Understanding Autoimmune Disorders: Causes, Symptoms, and Effective Management
Introduction
A. Definition of Autoimmune Diseases
When we talk about the immune system, most people picture a vigilant army that protects the body from invading pathogens. For millions of individuals, however, that army turns on the very host it’s meant to defend. Autoimmune diseases are a broad group of conditions in which the immune system—our body’s natural defense against infection—mistakenly attacks its own healthy tissues. Instead of targeting viruses, bacteria, or other foreign invaders, immune cells produce antibodies that recognize and damage organs, joints, skin, glands, or blood vessels. In essence, the immune system “turns on” itself, leading to chronic inflammation and tissue injury.
“Autoimmunity is not a single disease; it is a spectrum of disorders that share a common underlying malfunction of immune regulation.” – Dr. Emily Carter, Immunology Professor, University of California, San Diego
B. Prevalence and Impact on Individuals
More than 80 million Americans—roughly 1 in 3 people—live with an autoimmune disorder, according to the American Autoimmune Related Diseases Association (AARDA). Women are disproportionately affected, comprising about 78 % of all cases. The impact goes far beyond physical symptoms: chronic pain, fatigue, and unpredictable flare‑ups can erode quality of life, limit work productivity, and strain mental health. Early recognition and comprehensive management are therefore critical for preserving both health and independence.
“Autoimmune disease is not a single illness; it’s a family of disorders that demand a family‑like approach—education, empathy, and coordinated care.”
— Dr. Maya Patel, MD, Immunology Fellow, Mayo Clinic
In this post we will explore how these disorders arise, the hallmark signs you should look out for, and the blend of natural and medical strategies that can help us (and those we care for) regain control.
I. Understanding Autoimmune Diseases
A. Causes of Autoimmune Diseases
The exact trigger for most autoimmune diseases remains elusive, but research points to a multifactorial origin—a combination of genetics, environmental exposures, and lifestyle factors.
| Factor | How It Contributes | Example |
| Genetic predisposition | Certain HLA (human leukocyte antigen) genes increase susceptibility. | HLA‑DR4 is linked to rheumatoid arthritis. |
| Infections | Molecular mimicry: pathogens share structural elements with self‑proteins, confusing the immune system. | Epstein‑Barr virus is implicated in lupus and multiple sclerosis. |
| Hormonal influences | Estrogen can modulate immune activity, partially explaining higher female prevalence. | Women experience flare‑ups of systemic lupus erythematosus (SLE) during pregnancy. |
| Environmental toxins | Heavy metals, silica dust, and certain solvents can trigger dysregulated immunity. | Silica exposure is associated with systemic sclerosis. |
| Gut microbiome imbalance | Dysbiosis can shift immune responses toward autoimmunity. | Low diversity of Faecalibacterium prausnitzii linked to inflammatory bowel disease (IBD). |
| Stress and trauma | Chronic psychosocial stress can amplify inflammatory cytokines. | High perceived stress correlates with increased disease activity in rheumatoid arthritis. |
A.1 Genetic Predisposition
- Family history is one of the strongest risk factors. Certain HLA (human leukocyte antigen) genes increase susceptibility to diseases such as rheumatoid arthritis, type 1 diabetes, and Hashimoto’s thyroiditis.
- Twin studies reveal that if one identical twin has an autoimmune disease, the other has a 30‑50 % chance of developing a related condition.
- .2 Environmental Influences
| Environmental Factor | How It May Contribute | Examples |
| Infections | Molecular mimicry – pathogens share structural motifs with self‑proteins, confusing the immune system. | Epstein‑Barr virus (linked to lupus), Helicobacter pylori (linked to autoimmune gastritis) |
| Smoking | Alters lung tissue, triggers citrullination of proteins → rheumatoid arthritis. | Cigarette smoke, vaping |
| Dietary components | Gluten, dairy, and certain food additives can provoke gut permeability (“leaky gut”). | Celiac disease, non‑celiac gluten sensitivity |
| Chemical exposures | Industrial solvents, silica dust, and certain pesticides can trigger autoimmune reactivity. | Silica exposure in mining → systemic sclerosis |
| Stress & trauma | Chronic stress dysregulates cortisol, impairing immune tolerance. | High‑stress occupations, major life events |
A .3 Hormonal and Gender Factors
- Women are disproportionately affected—about 78 % of diagnosed cases occur in women. Estrogen may amplify immune activity, while progesterone can have a protective effect.
- Pregnancy can temporarily improve symptoms of some diseases (e.g., rheumatoid arthritis) but worsen others (e.g., systemic lupus erythematosus).
A .4 Gut Microbiome
- A balanced gut microbiota educates the immune system. Dysbiosis—an imbalance of beneficial vs. harmful bacteria—has been linked to inflammatory bowel disease, multiple sclerosis, and type 1 diabetes.
- “We now understand that the gut is an immune organ; when the microbial community is disturbed, autoimmunity can flourish.” – Prof. Marco Liu, Microbiome Research Center.
Most patients carry a genetic risk but never develop disease—environmental “hits” are usually required to set the auto‑reactive cascade in motion.
B. Mechanism of Autoimmune Response: A Step-by-Step Breakdown.
Autoimmune diseases arise from a fundamental breakdown in the body’s ability to distinguish self from non-self, resulting in an immune attack on its own tissues. This process, known as a loss of self-tolerance, unfolds through a series of interconnected immunological events involving both innate and adaptive immunity. Understanding the mechanism of autoimmune response is essential for diagnosing, managing, and developing targeted therapies for conditions such as rheumatoid arthritis, lupus, type 1 diabetes, and Hashimoto’s thyroiditis.
**Loss of Self-Tolerance**
Under normal conditions, the immune system undergoes rigorous education to prevent autoimmunity. During development in the primary lymphoid organs—specifically the thymus for T cells and the bone marrow for B cells—autoreactive lymphocytes that strongly recognize self-antigens are typically eliminated through a process called central tolerance. However, this process is not perfect, and some autoreactive T and B cells escape into the periphery. To prevent these rogue cells from causing harm, multiple peripheral tolerance mechanisms exist. Regulatory T cells (Tregs) play a crucial role by suppressing the activation and proliferation of autoreactive lymphocytes. Other mechanisms include anergy (functional inactivation of self-reactive cells upon antigen encounter without co-stimulation) and immune checkpoint pathways (e.g., CTLA-4, PD-1), which act as “brakes” on immune activation. When these regulatory systems fail—due to genetic predisposition, environmental triggers, or both—autoreactive cells can become activated and initiate an autoimmune cascade.
**Activation of Autoreactive Lymphocytes**
The transition from immune tolerance to autoimmunity often requires a triggering event. Common triggers include infections (via molecular mimicry, where microbial antigens resemble self-proteins), tissue injury (releasing sequestered antigens), or environmental factors like UV radiation or certain drugs. In an inflammatory context, antigen-presenting cells (such as dendritic cells) engulf self-antigens and present them to T cells in lymph nodes, providing both antigen-specific signals and co-stimulatory molecules. This aberrant activation overcomes the usual safeguards, leading to the proliferation of autoreactive T helper (Th) cells, particularly Th1 and Th17 subsets, which drive inflammation.
**Cytokine Storm and Chronic Inflammation**
Once activated, autoreactive T cells release a surge of pro-inflammatory cytokines, including interleukin-1 (IL-1), IL-6, and tumor necrosis factor-alpha (TNF-α). These signaling molecules act as powerful amplifiers of the immune response: they increase vascular permeability, recruit neutrophils and macrophages to the site of inflammation, and stimulate further immune cell activation. This self-sustaining loop creates a “cytokine storm” that perpetuates tissue damage. Additionally, activated T cells provide help to autoreactive B cells, prompting them to differentiate into plasma cells that produce autoantibodies—antibodies directed against the body’s own proteins.
**Targeted Tissue Destruction**
Autoantibodies bind to specific self-antigens on the surface of cells or within tissues. For example, in Hashimoto’s thyroiditis, autoantibodies target thyroid peroxidase (TPO) and thyroglobulin, leading to complement activation, antibody-dependent cellular cytotoxicity (ADCC), and infiltration of immune cells into the thyroid gland. This results in gradual destruction of thyroid follicular cells, impaired hormone production, and clinical hypothyroidism. In other diseases, immune complexes deposit in tissues (e.g., kidneys in lupus nephritis), triggering inflammation and organ dysfunction.
The net result is persistent, often systemic inflammation that underlies the diverse and sometimes debilitating symptoms of autoimmune diseases. This understanding has paved the way for biologic therapies that target specific cytokines (e.g., anti-TNF agents) or immune cells, offering more precise and effective treatment options.
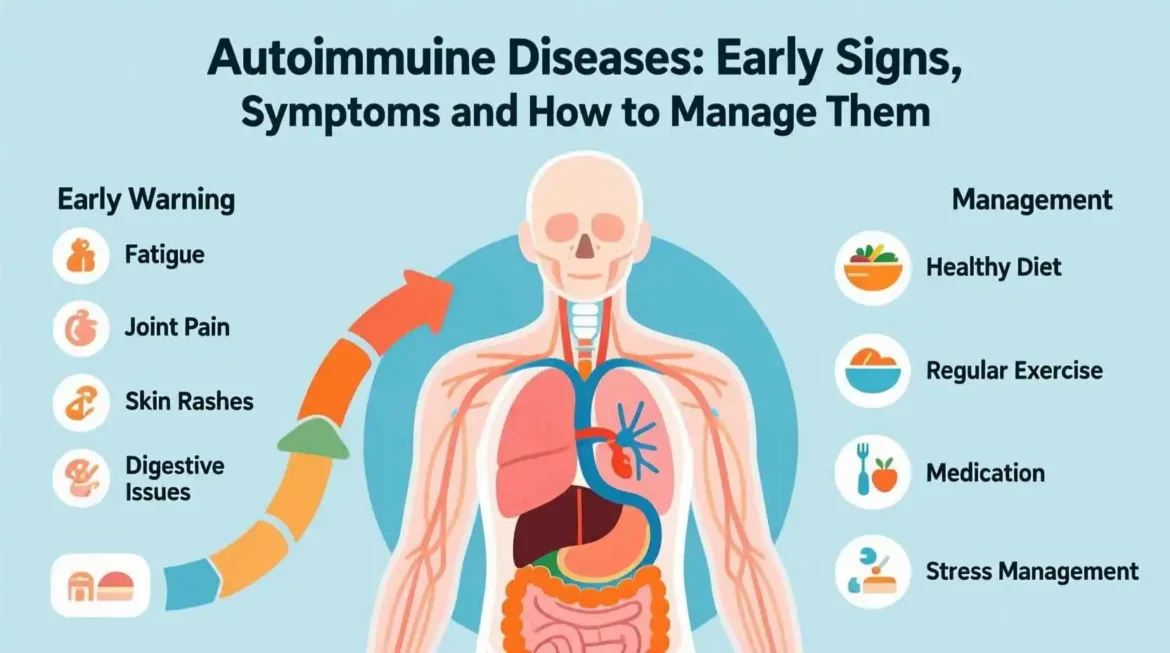
II. Symptoms of Autoimmune Diseases
A. Common Symptoms Across Different Autoimmune Diseases
Certain signs tend to recur across many disorders, making them useful “red flags” for early detection. Below is a concise list that incorporates many of our SEO keywords.
| Symptom | Why It Happens | Typical Frequency |
| Fatigue | Cytokine‑mediated metabolic slowdown; disrupted sleep from pain. | > 80 % of patients (e.g., lupus, Hashimoto’s). |
| Joint pain / swelling | Synovial inflammation from immune complex deposition. | Classic in rheumatoid arthritis, psoriatic arthritis. |
| Skin rashes | Auto‑antibodies attack skin cells; vasculitis. | “Butterfly” rash in lupus; guttate psoriasis in psoriatic disease. |
| Digestive issues | Gut‑associated lymphoid tissue inflammation; altered microbiome. | Diarrhea, bloating in Crohn’s disease, ulcerative colitis. |
| Unexplained weight loss | Increased basal metabolic rate, malabsorption. | Often seen in hyperthyroidism (Graves) and IBD. |
| Hair loss | Follicular inflammation; thyroid hormone imbalance. | Alopecia areata, Hashimoto’s, lupus. |
| Brain fog | Cytokine‑induced neuroinflammation; sleep disruption. | Common in multiple sclerosis, lupus, chronic fatigue syndrome. |
| Weak immune system symptoms (frequent infections) | Immune dysregulation can paradoxically impair pathogen defense. | Seen in systemic lupus and immunosuppressive therapy side‑effects. |
| Inflammation symptoms (fever, malaise) | Systemic release of IL‑6, TNF‑α. | Many autoimmune flares. |
“If you find yourself constantly exhausted, battling joint aches, and watching your skin change for no reason, it may be time to ask your doctor about an autoimmune work‑up.”
— Emily Rivera, patient advocate, LivingWithAutoimmune.org
B. Variability of Symptoms Depending on the Specific Disease
While the table above captures the shared landscape, the clinical picture can vary dramatically based on which organ(s) are targeted. Below we highlight three common diseases, illustrating both overlapping and disease‑specific signs.
| Disease | Core Symptom Set | Distinctive Features |
| Hashimoto’s thyroiditis | Fatigue, weight gain, cold intolerance, hair loss, joint aches | Elevated thyroid‑peroxidase (TPO) antibodies; enlarged, painless thyroid (goiter). |
| Systemic lupus erythematosus (SLE) | Fatigue, joint pain, rash, photosensitivity, kidney involvement | “Butterfly” malar rash; anti‑dsDNA antibodies; proteinuria. |
| Rheumatoid arthritis (RA) | Symmetrical joint pain, morning stiffness >1 hour, swelling | Rheumatoid factor (RF) and anti‑CCP antibodies; erosive changes on X‑ray. |
| Inflammatory bowel disease (IBD) – Crohn’s/Ulcerative colitis | Abdominal pain, diarrhea, rectal bleeding, weight loss | Skip lesions & granulomas in Crohn’s; continuous colonic inflammation in UC. |
| Multiple sclerosis (MS) | Vision problems, numbness, weakness, gait instability, cognitive fog | MRI lesions in CNS; oligoclonal bands in CSF. |
Because each disease can affect multiple systems, diagnostic delays are common. The average lag between symptom onset and formal diagnosis is 5–7 years for many autoimmune conditions, underscoring the importance of early recognition.
III. Natural Remedies for Autoimmune Diseases
Lifestyle modifications are not a substitute for prescribed medication, but they can soften the inflammatory fire and improve overall resilience. Below we organize evidence‑based natural strategies under three pillars.
A. Diet and Nutrition
| Strategy | Rationale | Practical Tips |
| Anti‑inflammatory diet (Mediterranean, Paleo‑Autoimmune) | High in omega‑3 fatty acids, antioxidants, polyphenols; low in refined sugars and processed oils. | Aim for fatty fish (salmon, sardines) 2×/week; extra‑virgin olive oil; colorful veggies; limit sugary drinks. |
| Gluten‑free trial (especially for Hashimoto’s, celiac‑associated autoimmunity) | Molecular mimicry between gluten peptides and thyroid tissue may exacerbate autoimmunity. | 4‑week elimination; monitor symptoms and antibodies. |
| Elimination of nightshades & lectins | Some patients report worsened joint pain after consuming tomatoes, peppers, or legumes. | Conduct a 2‑week rotation: remove then re‑introduce one group at a time. |
| Probiotic & prebiotic support | Restores gut microbiome diversity, reduces gut‑derived inflammation. | Yogurt, kefir, sauerkraut, or a high‑quality multi‑strain supplement (e.g., 10‑billion CFU). |
| Vitamin D optimization | Vitamin D modulates T‑reg cells; deficiency linked to higher disease activity. | Serum 25‑OH‑D goal 40‑60 ng/mL; sunlight 10‑15 min daily + 1,000–2,000 IU vitamin D3 supplement. |
| Adequate magnesium & selenium | Cofactors for antioxidant enzymes and thyroid hormone conversion. | Magnesium glycinate 200–400 mg nightly; Brazil nuts (2–3 per day) for selenium. |