The Link Between Maternal Stress and Fetal Brain Development
Introduction
The journey of pregnancy and early motherhood represents one of the most transformative periods in human life. During this time, the developing fetal brain undergoes remarkable growth and organization, establishing neural circuits that will influence a child’s cognitive, emotional, and social development throughout their lifetime. Concurrently, expectant and new mothers navigate profound physical, psychological, and social changes that can trigger significant stress responses. When this stress becomes overwhelming or traumatic, it may not only affect the mother’s wellbeing but also potentially alter the delicate trajectory of perinatal brain development.
The connection between maternal psychological experiences and fetal development represents one of the most compelling areas of developmental science. This intricate relationship operates through multiple biological pathways, including endocrine, immune, and autonomic nervous system signaling between mother and fetus. Understanding these mechanisms has profound implications for clinical practice, public health policy, and our fundamental conception of how early experiences shape human development.
This comprehensive exploration delves into the complex interplay between maternal stress or trauma and perinatal brain development. We will examine the biological mechanisms that transmit maternal experiences to the developing fetus, review the empirical evidence linking maternal stress with specific neurodevelopmental outcomes, consider protective factors that can mitigate these effects, and discuss implications for intervention and support. By synthesizing current research across multiple disciplines, we aim to provide a nuanced understanding of this critical developmental period and the ways in which supporting maternal wellbeing may foster optimal neurodevelopmental outcomes for children.
Defining Key Concepts
Before exploring the relationship between maternal stress and perinatal brain development, it is essential to establish clear definitions of the key concepts involved. These definitions will provide a foundation for understanding the complex interplay between maternal experiences and fetal development.
Maternal Stress
Maternal stress encompasses the psychological and physiological responses experienced by women during pregnancy and the postpartum period when they perceive challenges that exceed their coping resources. Stress during this period can be categorized in several ways:
Acute stress refers to time-limited responses to specific events, such as a medical emergency during pregnancy or a difficult childbirth experience. These events typically trigger immediate physiological responses including activation of the hypothalamic-pituitary-adrenal (HPA) axis and sympathetic nervous system.
Chronic stress represents ongoing psychological strain resulting from persistent challenges such as financial insecurity, relationship difficulties, or demanding work conditions. Unlike acute stress, chronic stress may lead to sustained alterations in maternal physiology that could have more profound effects on fetal development.
Perceived stress relates to a woman’s subjective appraisal of her circumstances as overwhelming or threatening, regardless of the objective nature of the stressor. This subjective experience may be particularly relevant for understanding individual differences in stress responses during pregnancy.
Physiological stress involves measurable biological changes including elevated cortisol levels, increased inflammatory markers, and autonomic nervous system activity. These physiological markers provide objective indicators of stress that may mediate effects on fetal development.
Maternal Trauma
Maternal trauma represents a distinct category of psychological experience that differs from everyday stress in several important ways:
Trauma is typically defined as exposure to events involving actual or threatened death, serious injury, or sexual violence, either directly experienced, witnessed, or learned about occurring to close family members or friends. During the perinatal period, trauma may include pregnancy loss, traumatic childbirth experiences, intimate partner violence, or other life-threatening events.
Post-Traumatic Stress Disorder (PTSD) is a clinical syndrome that may develop following trauma exposure, characterized by symptoms such as intrusive memories, avoidance of trauma-related stimuli, negative alterations in mood and cognition, and hyperarousal. Research suggests that approximately 3-4% of women meet criteria for PTSD during pregnancy, with higher rates among certain populations.
Birth trauma refers specifically to psychological trauma resulting from childbirth experiences that are perceived as threatening, frightening, or distressing. Studies indicate that up to one-third of women may experience their childbirth as traumatic, with approximately 1-6% developing PTSD as a result.
Complex trauma describes exposure to multiple, prolonged traumatic events, often of an interpersonal nature, such as childhood abuse or neglect. Women with histories of complex trauma may enter pregnancy with altered stress physiology and psychological vulnerabilities that can affect their perinatal experiences.
Perinatal Brain Development
The perinatal period encompasses the time from conception through the first year after birth, representing a phase of extraordinary brain development characterized by:
Neurogenesis – the proliferation of new neurons, which occurs most intensely during the first half of pregnancy but continues in specific brain regions throughout the perinatal period.
Neuronal migration – the movement of neurons to their appropriate positions in the developing brain, a process that occurs primarily during the first and second trimesters.
Synaptogenesis – the formation of connections between neurons, which accelerates dramatically during the third trimester and continues postnatally, reaching peak levels in the first year of life.
Myelination – the development of fatty sheaths around nerve fibers that facilitate efficient neural transmission, which begins in the second trimester and continues through childhood and adolescence.
Apoptosis – programmed cell death that eliminates excess neurons and refines neural circuits, a process that is particularly active during late prenatal and early postnatal development.
These processes occur in a highly orchestrated sequence, with different brain regions developing according to specific timetables. This temporal and regional specificity means that the impact of maternal stress or trauma may vary depending on the timing of exposure during pregnancy.
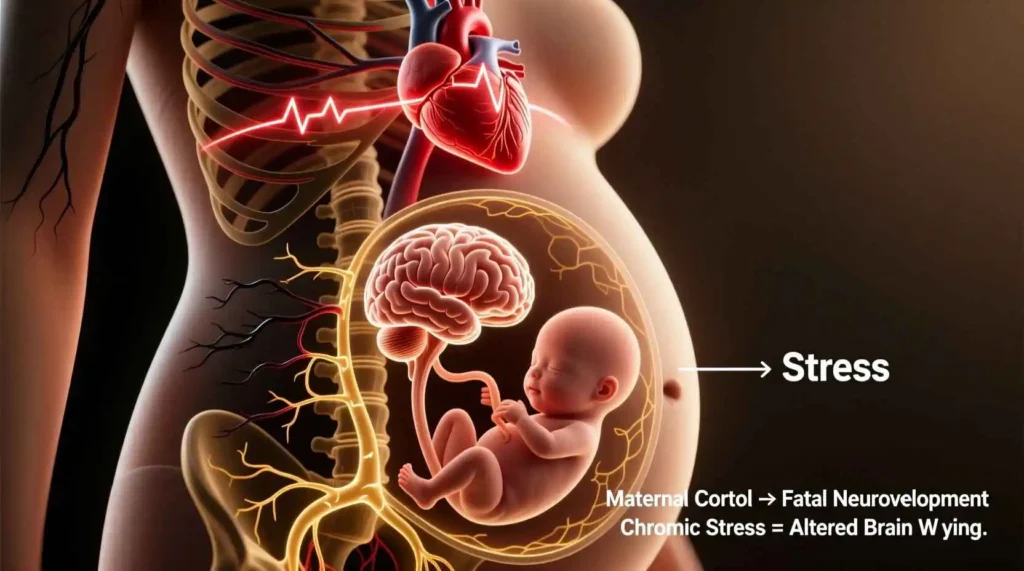
Biological Mechanisms Linking Maternal Stress to Fetal Brain Development
The connection between maternal psychological experiences and fetal development operates through multiple interconnected biological pathways. Understanding these mechanisms provides insight into how maternal stress or trauma might influence the developing brain.
The Hypothalamic-Pituitary-Adrenal (HPA) Axis
The HPA axis represents one of the primary pathways through which maternal stress may affect fetal development. This neuroendocrine system coordinates the body’s response to stress through a cascade of hormonal signals:
Corticotropin-Releasing Hormone (CRH): Produced in the hypothalamus, CRH stimulates the pituitary gland to release adrenocorticotropic hormone (ACTH). During pregnancy, the placenta also produces CRH, which increases exponentially across gestation and plays a role in determining the timing of delivery.
Adrenocorticotropic Hormone (ACTH): Released from the pituitary gland in response to CRH, ACTH stimulates the adrenal glands to produce cortisol.
Cortisol: The primary glucocorticoid hormone in humans, cortisol mobilizes energy resources and modulates immune function in response to stress. During pregnancy, maternal cortisol levels increase by two to four times compared to non-pregnant levels.
Under normal circumstances, the placenta serves as a partial barrier to maternal cortisol, converting approximately 80-90% to inactive cortisone through the enzyme 11β-hydroxysteroid dehydrogenase type 2 (11β-HSD2). However, this protective mechanism has limits:
- High levels of maternal stress can overwhelm the placental barrier’s capacity to inactivate cortisol
- The placental expression of 11β-HSD2 may be reduced by certain stressors, inflammation, or nutritional factors
- Placental CRH production is stimulated by maternal cortisol, creating a positive feedback loop that may further increase fetal exposure to glucocorticoids
When maternal cortisol crosses the placenta, it can influence fetal brain development through several mechanisms:
- Altered neurogenesis: Excess glucocorticoids may reduce the proliferation of new neurons, particularly in the hippocampus, a brain region critical for learning and memory.
- Impaired neuronal migration: Glucocorticoids can interfere with the movement of neurons to their appropriate positions during development, potentially disrupting cortical organization.
- Accelerated maturation: Prenatal exposure to elevated glucocorticoids may accelerate certain aspects of brain development, leading to earlier synaptic pruning and myelination that could alter neural circuitry.
- Epigenetic modifications: Glucocorticoids can induce epigenetic changes in the fetal brain, including DNA methylation and histone modifications that alter gene expression patterns long-term.
- HPA axis programming: Exposure to elevated glucocorticoids during sensitive periods may permanently alter the setpoint of the fetal HPA axis, leading to exaggerated stress responses throughout life.
Immune and Inflammatory Pathways
Maternal stress and trauma can also affect fetal brain development through immune and inflammatory mechanisms. The relationship between psychological stress and immune function is bidirectional and complex:
Stress-induced inflammation: Psychological stress can activate inflammatory pathways through several mechanisms:
- Sympathetic nervous system activation: Stress triggers the release of norepinephrine and epinephrine, which can bind to receptors on immune cells and stimulate the production of pro-inflammatory cytokines.
- Glucocorticoid receptor resistance: Chronic stress can reduce immune cells’ sensitivity to cortisol’s anti-inflammatory effects, leading to enhanced inflammatory responses.
- Cellular aging: Stress may accelerate immune cell aging through telomere shortening, potentially compromising immune regulation.
Maternal inflammation and fetal brain development: When maternal inflammation occurs during pregnancy, it can affect the fetal brain through several pathways:
- Cytokine transport: Pro-inflammatory cytokines such as interleukin-6 (IL-6) and tumor necrosis factor-alpha (TNF-α) can cross the placenta or activate placental inflammatory responses that affect the fetus.
- Microglial activation: Fetal microglia, the resident immune cells of the brain, are particularly sensitive to maternal inflammatory signals. Once activated, microglia release their own inflammatory molecules that can influence neuronal development and synaptic formation.
- Disruption of the blood-brain barrier: Inflammation may compromise the developing blood-brain barrier, increasing the fetal brain’s exposure to potentially harmful substances.
- Altered neurotransmitter systems: Inflammatory cytokines can affect the development of neurotransmitter systems, including serotonin and dopamine, which are critical for mood regulation and cognitive function.
Maternal immune activation (MIA): Research in animal models has demonstrated that experimentally induced maternal immune activation can lead to offspring with altered brain structure and function, including changes in cortical thickness, hippocampal volume, and white matter integrity. These findings have relevance for understanding how maternal stress-induced inflammation might affect human neurodevelopment.
Autonomic Nervous System and Cardiovascular Function
The autonomic nervous system (ANS) provides another pathway through which maternal stress may influence fetal development. The ANS regulates involuntary physiological functions including heart rate, blood pressure, and respiratory rate:
Sympathetic-Parasympathetic Balance: Stress activates the sympathetic branch of the ANS, triggering the “fight-or-flight” response, while reducing parasympathetic “rest-and-digest” activity. This imbalance can have several effects on the fetus:
- Reduced uterine blood flow: Sympathetic activation can cause vasoconstriction, potentially reducing blood flow to the uterus and placenta, thereby limiting oxygen and nutrient delivery to the fetus.
- Altered fetal heart rate patterns: Maternal stress responses can affect fetal heart rate variability and patterns, which may reflect changes in the developing autonomic nervous system.
- Placental function: ANS activity may influence placental function through effects on blood flow and hormone production.
Heart Rate Variability (HRV): HRV, the variation in time between heartbeats, serves as an index of autonomic flexibility and regulation. Lower maternal HRV during pregnancy has been associated with:
- Poorer fetal neurobehavioral development
- Increased risk of adverse birth outcomes
- Altered infant emotional and cognitive development
Vagal tone: The vagus nerve, a key component of the parasympathetic nervous system, plays a crucial role in regulating physiological responses to stress. Higher maternal vagal tone during pregnancy has been linked to better emotional regulation in infants, suggesting that maternal autonomic regulation may influence the development of offspring’s stress response systems.
Nutritional and Metabolic Pathways
Maternal stress and trauma can affect fetal brain development through alterations in nutrition and metabolism:
Nutrient Transfer: Stress may influence fetal development through effects on the placental transfer of critical nutrients:
- Reduced blood flow: As mentioned earlier, stress-induced vasoconstriction may limit the delivery of oxygen and nutrients to the placenta.
- Altered placental transporters: Stress hormones can affect the expression and function of placental nutrient transporters, potentially limiting the supply of amino acids, glucose, and fatty acids essential for brain development.
- Increased nutrient utilization: Maternal stress may increase maternal metabolic demands, potentially diverting nutrients away from the fetus.
Micronutrients: Several micronutrients critical for brain development may be affected by maternal stress:
- Iron: Stress may affect iron metabolism, and iron deficiency during pregnancy has been linked to altered neurodevelopment, including impaired myelination and neurotransmitter function.
- Folate: This B vitamin is essential for DNA synthesis and methylation, processes critical for neurodevelopment. Stress may affect folate metabolism and utilization.
- Omega-3 fatty acids: These fats, particularly docosahexaenoic acid (DHA), are crucial for brain development. Maternal stress may influence the placental transfer and metabolism of these essential fatty acids.
Metabolic Programming: Maternal stress can affect fetal metabolic programming through several mechanisms:
- Glucose metabolism: Stress hormones influence glucose regulation, and alterations in maternal glucose availability can affect fetal brain development.
- Insulin-like Growth Factors (IGFs): These hormones play important roles in brain development and may be affected by maternal stress.
- Leptin and other adipokines: These hormones regulate energy balance and have been implicated in neurodevelopment. Maternal stress may influence their production and signaling.
Epigenetic Mechanisms
Epigenetic modifications represent one of the most compelling mechanisms through which maternal stress might produce lasting effects on offspring brain development. Epigenetics refers to changes in gene expression that do not involve alterations to the DNA sequence itself:
DNA Methylation: The addition of methyl groups to DNA molecules, typically at cytosine-guanine dinucleotide (CpG) sites, can influence gene expression:
- Gene silencing: Methylation of promoter regions typically suppresses gene expression by making DNA less accessible to transcription machinery.
- Stress-induced methylation changes: Maternal stress has been associated with altered DNA methylation patterns in offspring, particularly in genes related to stress regulation, such as the glucocorticoid receptor gene (NR3C1).
- Tissue specificity: Epigenetic modifications may occur in a tissue-specific manner, with brain regions showing distinct patterns of methylation in response to prenatal stress.
Histone Modifications: Histones are proteins around which DNA is wrapped, and their modification can influence gene expression:
- Acetylation: Generally associated with increased gene expression by loosening chromatin structure.
- Methylation: Can either activate or repress gene expression depending on the specific histone and location of the methyl group.
- Stress effects: Maternal stress may alter histone modifications in the fetal brain, potentially affecting the expression of genes involved in neurodevelopment and stress regulation.
Non-coding RNAs: These RNA molecules do not code for proteins but can regulate gene expression:
- MicroRNAs (miRNAs): Small non-coding RNAs that can bind to messenger RNA and prevent protein production. Maternal stress may alter miRNA expression in the placenta and fetal brain.
- Other non-coding RNAs: Including long non-coding RNAs and piwi-interacting RNAs, which may also be influenced by maternal stress and affect neurodevelopment.
Transgenerational Effects: Some epigenetic modifications induced by prenatal stress may be transmitted across generations:
- Germline transmission: Epigenetic marks in germ cells (eggs or sperm) may escape the normal process of epigenetic erasure during fertilization, potentially affecting subsequent generations.
- Behavioral transmission: Stress-induced alterations in maternal behavior may affect offspring development and their subsequent parenting behaviors, creating intergenerational cycles.
- Critical periods: Epigenetic modifications may be particularly likely to occur during sensitive periods of development, such as the perinatal period, when epigenetic patterns are being established.
Timing and Duration of Maternal Stress Exposure
The impact of maternal stress on fetal brain development appears to depend significantly on the timing and duration of exposure. Different brain regions and developmental processes follow specific timetables, creating windows of vulnerability when stress may have particularly pronounced effects.
First Trimester
The first trimester (conception to 12 weeks gestation) represents a period of extraordinary cellular proliferation and early organization:
Early Developmental Processes: During this period, the neural tube forms and closes, and the basic structure of the brain begins to emerge. Key processes include:
- Neural induction: The process by which ectodermal cells are specified to become neural tissue
- Neurulation: The formation of the neural tube, which gives rise to the brain and spinal cord
- Early neurogenesis: The production of neurons that will populate the developing brain
- Neural crest cell migration: These cells give rise to diverse structures including parts of the peripheral nervous system
Potential Effects of First Trimester Stress: Research suggests that stress during this early period may affect:
- Risk of neural tube defects: Some studies have found associations between maternal stress and increased risk of structural abnormalities, although findings are not entirely consistent.
- Altered cell proliferation: Stress hormones may influence the rate of cell division in the developing brain, potentially affecting overall brain size or regional volumes.
- Epigenetic programming: Early gestation represents a critical period for epigenetic establishment, and stress during this time may induce particularly stable epigenetic modifications.
- Miscarriage risk: Severe stress during the first trimester has been associated with increased risk of pregnancy loss, possibly through effects on HPA axis function and immune responses.
Methodological Challenges: Studying the effects of first-trimester stress presents unique challenges:
- Timing of pregnancy recognition: Many women do not recognize their pregnancy until several weeks after conception, making it difficult to capture stress exposure during the earliest stages.
- Recall bias: When stress is assessed retrospectively, women’s reports of early pregnancy stress may be influenced by subsequent pregnancy experiences or outcomes.
- Selective survival: Severe stress effects may result in pregnancy loss, meaning that studies of live births may underestimate the true impact of early stress.
Second Trimester
The second trimester (13-28 weeks gestation) is characterized by continued growth and elaboration of brain structures:
Developmental Processes: Key events during this period include:
- Neuronal migration: The movement of neurons to their final positions in the developing brain
- Early synaptogenesis: The formation of initial connections between neurons
- Glia production: The generation of non-neuronal cells that support and protect neurons
- Regional specialization: The differentiation of distinct brain regions with specialized functions
Potential Effects of Second Trimester Stress: Stress during this period may influence:
- Neuronal migration: Animal studies suggest that prenatal stress can disrupt the normal migration of neurons, potentially leading to altered cortical organization.
- Gray matter development: Some human studies have found associations between second-trimester stress and reduced gray matter volume in specific brain regions.
- Cerebellar development: The cerebellum undergoes significant development during this period and may be particularly vulnerable to stress effects.
- Sensory system development: The basic organization of sensory systems occurs during this time, potentially making them sensitive to stress effects.
Sex-Specific Effects: Emerging evidence suggests that the effects of second-trimester stress may differ for male and female fetuses:
- Differential vulnerability: Some studies indicate that male fetuses may be more vulnerable to the effects of maternal stress during this period.
- Different patterns of effects: Stress may affect different brain regions or functions in males versus females, possibly related to sex-specific patterns of brain development or hormonal influences.
Third Trimester



