Albumin and Health: How This Multitasking Protein Protects Your Body
Introduction
In the intricate symphony of human physiology, few proteins play as many diverse and critical roles as albumin. This remarkable molecule, often overlooked in discussions about health and disease, serves as the unsung hero of our bloodstream, performing countless essential functions that sustain life itself. From maintaining fluid balance to transporting vital nutrients and hormones, albumin stands as a testament to the elegant complexity of biological systems. As we delve into the world of this abundant multitasker, we’ll uncover the fascinating story of how a single protein can influence so many aspects of human health, why its levels serve as crucial indicators in medical diagnostics, and how its therapeutic applications continue to evolve in modern medicine. This comprehensive exploration will reveal why albumin deserves far more attention than it typically receives, both in scientific circles and public awareness.
What is Albumin?
Albumin is the most abundant protein in human blood plasma, accounting for approximately 50-60% of the total plasma protein content. Synthesized primarily in the liver, this water-soluble protein belongs to a family of proteins known as albumins, which are characterized by their high solubility in water and moderate heat stability. The human body produces about 10-15 grams of albumin daily, maintaining a delicate balance between synthesis and degradation to sustain plasma concentrations typically ranging from 3.5 to 5.0 grams per deciliter.
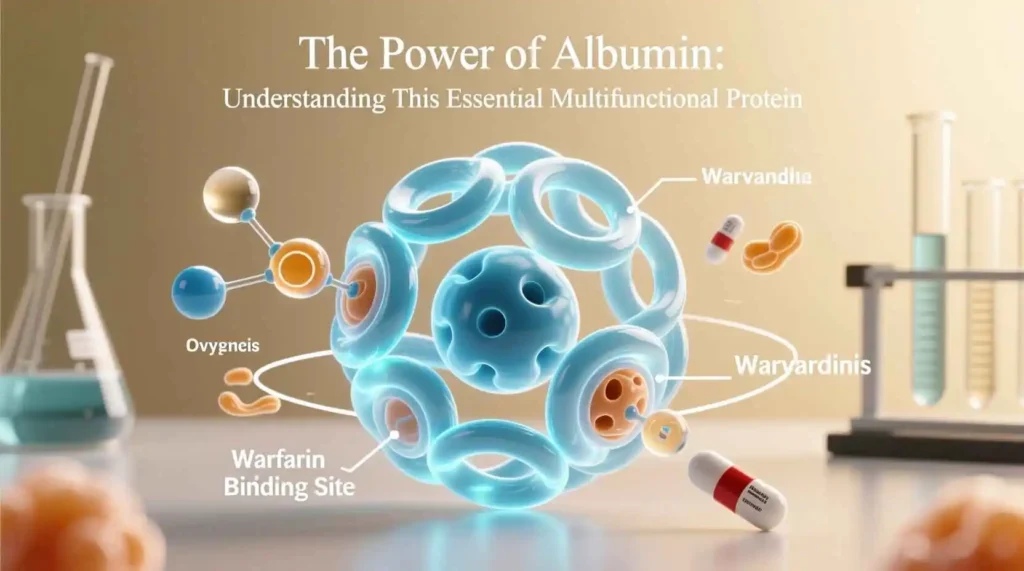
Chemically, albumin is a relatively small protein with a molecular weight of approximately 66.5 kilodaltons. Its structure consists of a single polypeptide chain of 585 amino acids, arranged in a heart-shaped conformation stabilized by 17 disulfide bonds. This unique three-dimensional structure creates multiple binding sites that allow albumin to interact with a vast array of molecules, making it one of the most versatile transporters in the human body.
The name “albumin” derives from the Latin word “albus,” meaning white, reflecting its appearance when isolated from egg whites (which contain a different but related albumin protein). In human physiology, albumin exists primarily in the intravascular space (within blood vessels), though a small portion can be found in interstitial fluids. Its presence is not limited to humans; albumin-like proteins are found throughout the animal kingdom, underscoring its fundamental biological importance.
Albumin’s half-life in circulation is remarkably long for a plasma protein, averaging about 20 days. This longevity allows it to perform its functions consistently without requiring constant replenishment. However, in certain disease states or nutritional deficiencies, albumin levels can drop significantly, leading to a cascade of physiological consequences that highlight its indispensable role in maintaining health.
Structure and Synthesis of Albumin
The molecular architecture of albumin represents a masterpiece of biological engineering. Its primary structure consists of a single chain of 585 amino acids, with a high proportion of charged residues that contribute to its solubility and functionality. The secondary structure features a series of alpha-helices that account for about 67% of its conformation, while the remaining structure consists of extended loops and turns that create its characteristic binding pockets.
The tertiary structure of albumin is particularly fascinating, forming a heart-shaped molecule with three homologous domains (I, II, and III), each divided into two subdomains (A and B). This arrangement creates nine loops connected by disulfide bonds, resulting in a stable yet flexible structure. The disulfide bonds are crucial for maintaining albumin’s structural integrity, as they prevent denaturation under physiological conditions and protect the protein from degradation.
Albumin’s synthesis occurs exclusively in the hepatocytes of the liver, where it is produced as preproalbumin. This precursor undergoes a series of modifications before becoming the mature protein found in circulation. Initially, the endoplasmic reticulum removes a signal peptide, converting preproalbumin to proalbumin. The Golgi apparatus then cleaves a hexapeptide from proalbumin, resulting in the mature albumin molecule that is secreted into the bloodstream.
The regulation of albumin synthesis is a complex process influenced by multiple factors. Nutritional status, particularly protein and calorie intake, plays a significant role, with adequate nutrition promoting synthesis while malnutrition suppresses it. Hormonal factors also contribute, with insulin, cortisol, and thyroid hormones stimulating albumin production, while inflammatory cytokines like interleukin-6 (IL-6) and tumor necrosis factor-alpha (TNF-α) can inhibit it. Additionally, oncotic pressure and plasma albumin concentration itself provide feedback mechanisms that modulate synthesis rates.
The gene responsible for albumin production, ALB, is located on chromosome 4 in humans and spans about 16,961 base pairs. This gene contains 15 exons that code for the albumin protein, with regulatory elements that control its expression in response to various physiological signals. Mutations in the ALB gene can lead to rare conditions such as familial dysalbuminemic hyperthyroxinemia or analbuminemia, though these are uncommon in the general population.
Once synthesized and secreted, albumin enters the bloodstream where it performs its myriad functions. Its distribution is primarily intravascular, with about 40% located in the extravascular space at any given time. This distribution is dynamic, with albumin constantly moving between compartments via the lymphatic system and capillary walls, maintaining equilibrium between intravascular and interstitial spaces.
Functions of Albumin
Albumin’s versatility as a multitasking protein stems from its ability to perform numerous physiological functions simultaneously. These roles are critical for maintaining homeostasis and supporting overall health, making albumin indispensable to human survival.
Osmotic Regulation
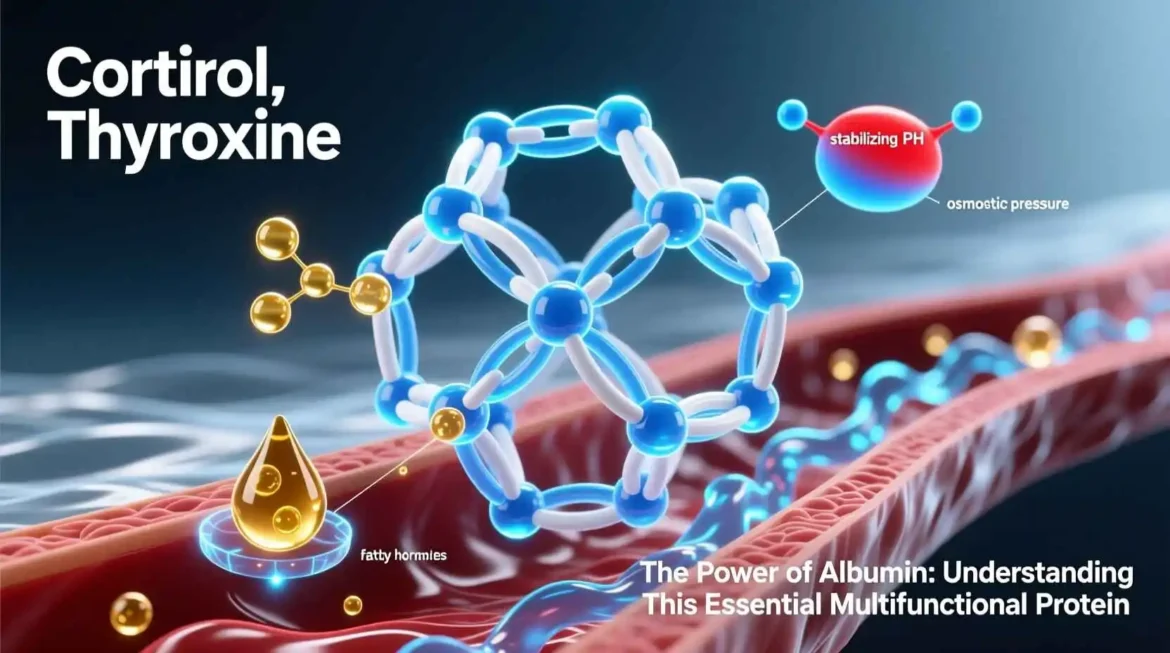
One of albumin’s most vital functions is maintaining colloidal osmotic pressure (also known as oncotic pressure) in the bloodstream. As the most abundant plasma protein, albumin accounts for approximately 75-80% of the total oncotic pressure. This pressure is essential for retaining fluid within the vascular compartment and preventing excessive leakage into interstitial spaces. The mechanism involves albumin’s negative charge attracting positively charged ions, which in turn hold water molecules within the blood vessels. Without adequate albumin, fluid would accumulate in tissues, leading to edema—a condition characterized by swelling, particularly in the extremities and abdomen.
Transport Functions
Albumin serves as the primary transport vehicle for numerous substances in the blood, thanks to its multiple binding sites and flexible structure. Its transport capabilities include:
- Fatty Acids: Albumin binds free fatty acids released from adipose tissue, transporting them to tissues for energy production or storage. Each albumin molecule can carry multiple fatty acid molecules simultaneously.
- Hormones: Many hormones, including thyroxine (T4), triiodothyronine (T3), cortisol, and aldosterone, bind to albumin for transport through the bloodstream. This binding protects hormones from degradation and helps regulate their bioavailability.
- Bilirubin: Albumin binds unconjugated bilirubin, a toxic breakdown product of heme, facilitating its transport to the liver for conjugation and excretion. This binding prevents bilirubin from crossing the blood-brain barrier and causing neurological damage.
- Metal Ions: Albumin transports essential metal ions such as calcium, copper, and zinc, maintaining their solubility and preventing toxic accumulation. For instance, about 40-45% of circulating calcium is bound to albumin.
- Drugs: Many pharmaceutical compounds, including antibiotics, anticoagulants, and anti-inflammatory drugs, bind to albumin. This binding affects drug distribution, metabolism, and elimination, influencing therapeutic efficacy and dosing requirements.
Antioxidant Properties
Albumin possesses significant antioxidant capabilities that protect tissues from oxidative damage. Its free thiol group at cysteine-34 acts as a potent scavenger of reactive oxygen species (ROS) and reactive nitrogen species (RNS). Additionally, albumin binds transition metals like copper and iron, preventing them from catalyzing free radical reactions through Fenton chemistry. These antioxidant functions are particularly important in conditions of oxidative stress, such as inflammation, ischemia-reperfusion injury, and chronic diseases.
Buffering Capacity
Albumin contributes to the body’s acid-base balance through its buffering capacity. With an isoelectric point of 4.7, albumin carries a net negative charge at physiological pH, allowing it to bind hydrogen ions and help maintain blood pH within the narrow range required for optimal cellular function. This buffering action becomes especially important in conditions of acidosis or alkalosis, where albumin helps mitigate pH fluctuations.
Binding and Detoxification
Albumin plays a crucial role in binding and detoxifying various harmful substances. It binds endogenous toxins such as bilirubin and bile acids, as well as exogenous toxins including heavy metals and environmental pollutants. By sequestering these substances, albumin reduces their bioavailability and facilitates their elimination from the body. This detoxification function is particularly important in liver disease, where impaired hepatic function compromises the body’s natural detoxification pathways.
Endothelial Function and Vascular Integrity
Emerging research has revealed that albumin contributes to endothelial function and vascular integrity. It helps maintain the glycocalyx—a protective layer on the endothelial surface—and supports nitric oxide signaling, which regulates vascular tone and blood flow. Albumin also binds and stabilizes sphingosine-1-phosphate, a lipid mediator that enhances endothelial barrier function and reduces vascular permeability.
Immunomodulatory Effects
Albumin exhibits immunomodulatory properties that influence both innate and adaptive immune responses. It can bind and neutralize pro-inflammatory substances, modulate leukocyte activation, and affect cytokine production. In conditions of systemic inflammation, such as sepsis, albumin’s immunomodulatory functions may help regulate excessive immune responses and prevent tissue damage.
Nutritional Marker
Albumin serves as a reliable marker of nutritional status due to its sensitivity to protein and calorie intake. Prolonged malnutrition leads to decreased albumin synthesis, resulting in hypoalbuminemia. However, because albumin has a long half-life, it reflects chronic rather than acute nutritional changes. Healthcare providers often use albumin levels alongside other markers to assess patients’ nutritional status and guide interventions.
Clinical Significance of Albumin
Albumin’s multifaceted roles in human physiology make it a critical parameter in clinical medicine. Its measurement provides valuable insights into various disease states, and its therapeutic applications continue to evolve. Understanding the clinical significance of albumin is essential for healthcare providers across multiple specialties.
Albumin as a Diagnostic Marker
Albumin levels are routinely measured as part of standard blood tests, providing clinicians with important diagnostic and prognostic information. Hypoalbuminemia (low albumin levels) is associated with numerous pathological conditions, including:
- Liver Disease: Since albumin is synthesized in the liver, impaired hepatic function in conditions like cirrhosis, hepatitis, or liver failure leads to decreased production. Albumin levels correlate with disease severity and prognosis in chronic liver disease.
- Kidney Disease: In nephrotic syndrome, damaged glomeruli allow albumin to leak into urine, resulting in hypoalbuminemia. The degree of proteinuria often correlates with disease progression and response to treatment.
- Malnutrition: Inadequate protein or calorie intake suppresses albumin synthesis. Hypoalbuminemia is common in protein-energy malnutrition, anorexia nervosa, and cachexia associated with chronic diseases.
- Inflammatory Conditions: Acute and chronic inflammation increases vascular permeability and shifts albumin from intravascular to interstitial spaces. Cytokines also suppress albumin synthesis, contributing to hypoalbuminemia in conditions like sepsis, trauma, and autoimmune disorders.
- Burns: Extensive burns cause massive fluid loss and increased capillary permeability, leading to rapid depletion of circulating albumin.
- Gastrointestinal Disorders: Conditions like protein-losing enteropathy, inflammatory bowel disease, and malabsorption syndromes can cause hypoalbuminemia through reduced absorption or increased loss.
Hyperalbuminemia (elevated albumin levels) is less common but can occur in dehydration, where hemoconcentration artificially raises albumin concentration. It may also result from excessive intravenous albumin administration.
Albumin in Critical Care
In critical care settings, albumin levels serve as important prognostic indicators. Hypoalbuminemia upon admission to intensive care units (ICUs) is associated with increased mortality, longer hospital stays, and higher complication rates. This relationship holds true across various critical conditions, including sepsis, acute respiratory distress syndrome (ARDS), and major surgery.
The use of albumin as a volume expander in critical care has been extensively studied. While crystalloid solutions remain first-line for fluid resuscitation, albumin may be beneficial in specific scenarios:
- Hypovolemic Shock: Albumin can be used when crystalloids fail to restore adequate hemodynamics.
- Cirrhosis with Spontaneous Bacterial Peritonitis: Albumin infusion reduces renal impairment and mortality in these patients.
- Large-Volume Paracentesis: In cirrhotic patients with ascites, albumin administration after paracentesis prevents circulatory dysfunction.
- Acute Liver Failure: Albumin may improve outcomes by supporting circulatory function and reducing oxidative stress.
Albumin in Nephrology
In kidney disease, albumin plays a dual role as both a marker and a therapeutic agent. Urinary albumin excretion is a sensitive indicator of glomerular damage, serving as an early marker for diabetic nephropathy and other forms of chronic kidney disease (CKD). Microalbuminuria (30-300 mg/day) often precedes overt proteinuria and declining renal function.
For patients with nephrotic syndrome, hypoalbuminemia contributes to edema formation through reduced oncotic pressure. Management includes addressing the underlying cause, dietary modifications, and sometimes diuretic therapy. In severe cases, albumin infusions may be used transiently to improve symptoms, though they do not address the underlying pathology.
Albumin in Hepatology
In liver disease, albumin synthesis is impaired, leading to hypoalbuminemia that correlates with disease severity. The Child-Pugh score and Model for End-Stage Liver Disease (MELD) score—used to assess prognosis in cirrhosis—incorporate albumin levels as key parameters.
Albumin has several therapeutic applications in hepatology:
- Ascites Management: Albumin infusions prevent circulatory dysfunction after large-volume paracentesis in cirrhotic patients.
- Hepatorenal Syndrome: Albumin combined with vasoconstrictors improves renal function in this complication of advanced cirrhosis.
- Spontaneous Bacterial Peritonitis: Albumin reduces renal impairment and mortality in cirrhotic patients with this infection.
- Acute-on-Chronic Liver Failure: Albumin may improve outcomes by supporting circulatory function and reducing systemic inflammation.
Albumin in Cardiovascular Medicine
Albumin influences cardiovascular health through multiple mechanisms. Its role in maintaining oncotic pressure affects fluid balance and cardiac preload. Additionally, albumin binds nitric oxide, modulating its bioavailability and influencing vascular tone. In heart failure, hypoalbuminemia is associated with worse outcomes, including increased mortality and hospitalization rates.
Albumin infusions have been studied in cardiovascular conditions such as cardiogenic shock and cardiac surgery, though evidence for routine use remains limited. In specific scenarios like post-cardiopulmonary bypass, albumin may help maintain hemodynamic stability.
Albumin in Oncology
Cancer patients frequently develop hypoalbuminemia due to multiple factors, including reduced intake, increased metabolic demands, inflammation, and sometimes direct effects of tumors or treatments. Low albumin levels correlate with poor prognosis in various malignancies, reflecting both nutritional status and systemic inflammation.
In oncology practice, albumin serves as a component of prognostic scoring systems like the Glasgow Prognostic Score. Albumin infusions may be used in cancer patients with severe hypoalbuminemia, though they do not address the underlying causes and should be part of a comprehensive nutritional support strategy.
Albumin in Surgery and Perioperative Care
Preoperative albumin levels are strong predictors of surgical outcomes. Hypoalbuminemia is associated with increased postoperative complications, including wound infections, anastomotic leaks, and prolonged hospital stays. This relationship holds true across various surgical specialties, from general surgery to orthopedics.
In perioperative care, albumin may be used in specific situations:
- Major Abdominal Surgery: Albumin infusions may help maintain hemodynamic stability in patients with significant fluid shifts.
- Liver Resection: Albumin supports oncotic pressure and reduces complications in patients undergoing hepatectomy.
- Cardiac Surgery: Albumin may be used as part of priming solutions for cardiopulmonary bypass or for postoperative volume expansion.
Albumin in Nutrition Support
Albumin is commonly used as a marker of nutritional status, though its limitations must be recognized. Due to its long half-life and sensitivity to non-nutritional factors like inflammation, albumin reflects chronic rather than acute nutritional changes. It should be interpreted alongside other markers such as prealbumin (transthyretin), transferrin, and retinol-binding protein for a comprehensive nutritional assessment.
In nutritional support, albumin infusions are sometimes used in severely malnourished patients, though evidence for their efficacy is limited. Oral nutritional supplements, enteral nutrition, or parenteral nutrition are preferred strategies for improving nutritional status, with albumin levels serving as one indicator of response.
Albumin in Disease States
Albumin’s involvement in various disease states extends beyond its role as a mere biomarker. alterations in albumin structure, function, or concentration contribute to pathophysiology and influence disease progression. Understanding these relationships provides insights into disease mechanisms and potential therapeutic approaches.
Liver Disease