L-Arginine and L-Citrulline Supplements: Which One Should You Take for Heart Health, Energy & Performance
Introduction: The Nitric Oxide Revolution in Human Physiology
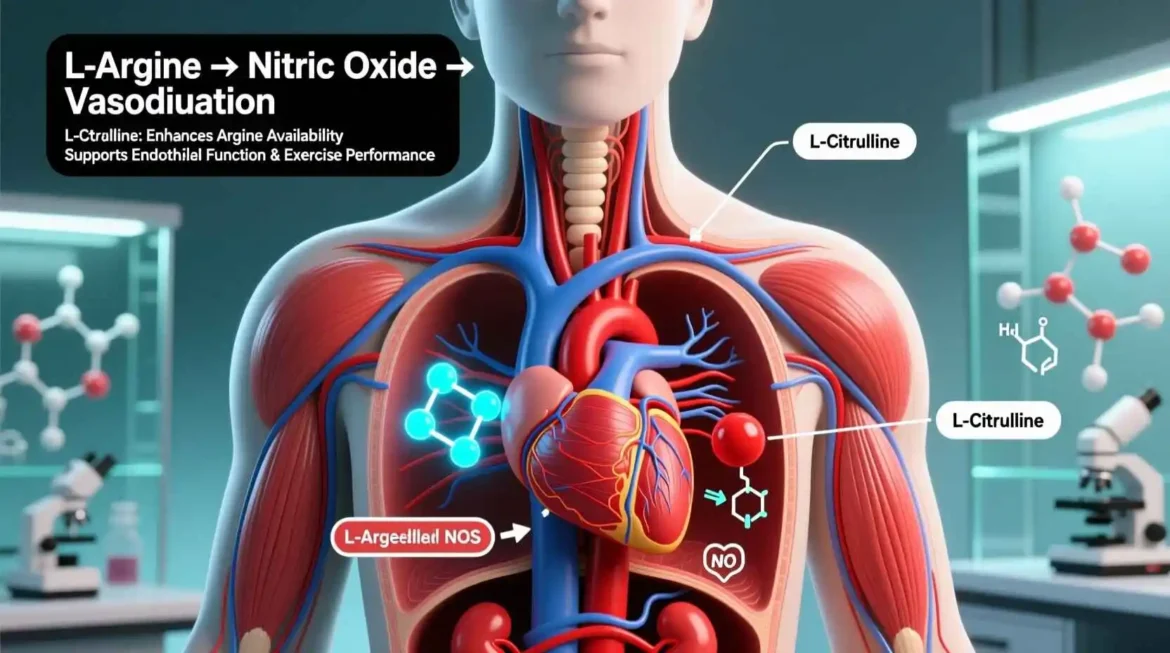
In the vast landscape of human biochemistry, few amino acid pairs have garnered as much scientific attention and clinical relevance as L-arginine and L-citrulline. These conditionally essential amino acids form the cornerstone of nitric oxide (NO) metabolism—a signaling pathway so fundamental to human health that its discovery earned the 1998 Nobel Prize in Physiology or Medicine. Nitric oxide, a simple diatomic gas, serves as the body’s most potent vasodilator, a critical neurotransmitter, and a key modulator of immune function. The intricate relationship between L-arginine and L-citrulline represents one of nature’s most elegant biochemical cycles, where each compound sustains the other’s availability for continuous NO production.
The significance of this amino acid pair extends far beyond their individual roles. L-arginine, discovered in 1886, was initially recognized for its involvement in urea detoxification. It took nearly a century to uncover its pivotal role as the exclusive substrate for nitric oxide synthase enzymes. L-citrulline, first isolated from watermelon in 1914, was long considered merely a metabolic byproduct until researchers revealed its crucial function in recycling back to L-arginine, thereby sustaining NO production far more efficiently than arginine supplementation alone.
Today, the therapeutic applications of L-arginine and L-citrulline span numerous medical disciplines. From cardiovascular medicine to sports performance, from metabolic health to reproductive function, these amino acids demonstrate remarkable versatility. Their ability to enhance endothelial function, improve blood flow, regulate cellular metabolism, and modulate immune responses has positioned them as frontline nutraceuticals in both preventive and therapeutic contexts.
This comprehensive exploration delves into every aspect of L-arginine and L-citrulline biochemistry, physiology, and clinical applications. We will examine their molecular structures, metabolic pathways, synergistic interactions, and evidence-based therapeutic uses across diverse health conditions. Special attention will be given to their combined supplementation strategies, safety profiles, and practical implementation guidelines. By understanding the full spectrum of their biological activities, healthcare practitioners and health-conscious individuals alike can harness the full potential of these remarkable compounds to optimize human health and performance.
1: Biochemical Foundations of L-Arginine and L-Citrulline
1.1 Molecular Structure and Chemical Properties
L-Arginine (2-amino-5-guanidinopentanoic acid) is a basic amino acid characterized by its distinctive guanidinium group. This α-amino acid contains an α-carbon atom bonded to an amino group, a carboxyl group, a hydrogen atom, and a three-carbon aliphatic side chain terminating in a guanidino group. The guanidinium group gives L-arginine its strong basic properties, with a pKa of approximately 12.5, making it positively charged at physiological pH. This positive charge facilitates its interactions with negatively charged molecules in biological systems, including DNA, RNA, and various proteins.
The molecular formula of L-arginine is C₆H₁₄N₄O₂, with a molecular weight of 174.20 g/mol. It exists as a white, water-soluble crystalline powder at room temperature. The L-configuration denotes its stereochemistry, which is biologically active in human systems. The guanidino group’s planar structure and resonance stabilization contribute to its unique biochemical reactivity, enabling its participation in multiple enzymatic reactions beyond protein synthesis.
L-Citrulline (2-amino-5-ureidopentanoic acid) shares structural similarities with L-arginine but contains a ureido group instead of a guanidinium group. Its molecular formula is C₆H₁₃N₃O₃, with a molecular weight of 175.19 g/mol. The name derives from its initial discovery in watermelon (Citrullus vulgaris). Unlike L-arginine, L-citrulline is not incorporated into proteins during ribosomal translation, which distinguishes its metabolic fate from standard amino acids.
The ureido group in L-citrulline consists of a carbonyl group bonded to two amine groups, giving it distinct chemical properties. This structure makes L-citrulline highly soluble in water and resistant to enzymatic degradation by arginase, a critical factor in its metabolic advantages. The absence of a charged group at physiological pH allows L-citrulline to traverse cellular membranes more freely than L-arginine, contributing to its superior pharmacokinetic profile.
1.2 Biosynthesis and Metabolic Pathways
Endogenous Production of L-Arginine The human body synthesizes L-arginine through the intestinal-renal axis, a sophisticated metabolic pathway involving multiple organs. The process begins in the small intestine, where epithelial cells convert glutamine to glutamate and then to ornithine via ornithine aminotransferase. Ornithine is then combined with carbamoyl phosphate to form citrulline, catalyzed by ornithine transcarbamylase. This citrulline enters the bloodstream and travels to the kidneys, where proximal tubule cells convert it back to L-arginine through a two-step process involving argininosuccinate synthase and argininosuccinate lyase.
This intestinal-renal axis is particularly important during periods of increased demand, such as growth, recovery from injury, or certain disease states. However, under normal conditions, endogenous production meets only approximately 60% of the body’s requirements, making dietary intake essential for optimal health. The rate-limiting step in this pathway is argininosuccinate synthase, which is subject to feedback regulation by L-arginine and other metabolites.
L-Citrulline Formation and Recycling L-Citrulline is produced both endogenously and obtained from dietary sources. The primary endogenous source is the nitric oxide synthase (NOS) pathway, where L-arginine is converted to nitric oxide and L-citrulline. This reaction occurs in endothelial cells, neurons, and immune cells, depending on the NOS isoform involved. Additionally, L-citrulline can be generated from glutamine via the citrulline-NO cycle in enterocytes.
The metabolic fate of L-citrulline is particularly interesting. Unlike most amino acids, it bypasses hepatic metabolism and is efficiently taken up by the kidneys. In renal proximal tubule cells, L-citrulline is converted to L-arginine through the citrulline-arginine cycle, involving argininosuccinate synthase and argininosuccinate lyase. This conversion efficiency approaches 80%, making L-citrulline an effective precursor for L-arginine replenishment. The kidneys then release this newly synthesized L-arginine into the systemic circulation, where it becomes available for NO production and other metabolic functions.
The Urea Cycle Connection Both amino acids play integral roles in the urea cycle, the primary pathway for nitrogen detoxification in mammals. L-Arginine acts as an allosteric activator of N-acetylglutamate synthase, which produces N-acetylglutamate, an essential activator of carbamoyl phosphate synthetase I—the rate-limiting enzyme of the urea cycle. Within the cycle itself, L-arginine is hydrolyzed by arginase to produce ornithine and urea. Ornithine then re-enters the cycle, combining with carbamoyl phosphate to form citrulline.
L-Citrulline serves as an intermediate in this cycle, formed from ornithine and carbamoyl phosphate. It then condenses with aspartate to form argininosuccinate, which is subsequently cleaved to yield L-arginine and fumarate. This intimate connection to the urea cycle highlights the importance of both amino acids in ammonia detoxification, particularly in liver disease and metabolic disorders where nitrogen accumulation becomes toxic.
1.3 Pharmacokinetics and Bioavailability
Absorption and Distribution L-Arginine absorption occurs primarily in the small intestine through specific cationic amino acid transporters (CATs), particularly CAT-1 and CAT-2B. These transporters have broad specificity but preferentially transport L-arginine, L-lysine, and L-ornithine. The absorption efficiency of oral L-arginine is approximately 60-70%, but this can be significantly reduced by several factors. First-pass metabolism in the liver extracts a substantial portion of absorbed L-arginine, with up to 40% being catabolized before reaching systemic circulation. Additionally, intestinal arginase enzymes degrade approximately 30-40% of ingested L-arginine before absorption, further limiting its bioavailability.
Once absorbed, L-arginine distributes widely throughout the body, with the highest concentrations found in the liver, kidneys, and skeletal muscle. It readily crosses the blood-brain barrier via specific transporters, allowing it to influence neurotransmitter systems. Plasma concentrations of L-arginine typically range from 80-150 μmol/L under normal conditions, with significant fluctuations based on dietary intake and metabolic demands.
L-Citrulline absorption occurs through passive diffusion and possibly via neutral amino acid transporters in the small intestine. Its bioavailability exceeds 90%, significantly higher than L-arginine, due to several key factors. Unlike L-arginine, L-citrulline is not a substrate for intestinal arginase, avoiding presystemic degradation. It also bypasses first-pass hepatic metabolism, as the liver lacks efficient transporters for citrulline uptake. Instead, L-citrulline is efficiently taken up by the kidneys via sodium-dependent transporters, where it undergoes conversion to L-arginine.
The distribution of L-citrulline is more limited than L-arginine, primarily confined to the vascular compartment and kidneys. However, its conversion to L-arginine in renal tissue allows for sustained elevation of plasma arginine levels for up to 8 hours after ingestion, compared to only 1-2 hours with direct L-arginine supplementation.
Metabolism and Elimination L-Arginine undergoes extensive metabolism through multiple pathways. Approximately 40% is catabolized by arginase to ornithine and urea, which enter the urea cycle. Another 5-10% serves as a substrate for nitric oxide synthase enzymes, producing NO and citrulline. Additional metabolic fates include creatine synthesis (combining with glycine and methionine), polyamine production (via ornithine decarboxylase), agmatine formation, and protein synthesis. The elimination half-life of L-arginine is approximately 1-2 hours, with metabolites primarily excreted renally.
L-Citrulline metabolism is more straightforward, with the primary pathway being conversion to L-arginine in the kidneys. A small portion (approximately 10-15%) may be converted to arginine in other tissues, including endothelial cells and enterocytes. Unlike L-arginine, L-citrulline is not significantly incorporated into proteins or metabolized by arginase. Its elimination half-life is approximately 45-60 minutes, but its effects on plasma arginine levels persist much longer due to sustained renal conversion.
Comparative Pharmacokinetics The pharmacokinetic differences between these amino acids have profound implications for their therapeutic use. L-Citrulline administration results in a more sustained elevation of plasma L-arginine levels compared to direct L-arginine supplementation. Studies show that a 3-gram dose of L-citrulline increases plasma arginine levels by approximately 200% for up to 8 hours, whereas a similar dose of L-arginine produces only a transient 60-70% increase lasting 1-2 hours. This prolonged elevation makes L-citrulline particularly effective for conditions requiring sustained NO production, such as cardiovascular support and exercise performance enhancement.
Additionally, L-citrulline avoids the “arginase trap” that limits L-arginine efficacy. Arginase enzymes, upregulated in many disease states including cardiovascular disease and diabetes, actively degrade L-arginine, reducing its availability for NO synthesis. L-Citrulline, being resistant to arginase, provides an alternative pathway to maintain arginine pools and support NO production under these pathological conditions.
2: Physiological Functions of L-Arginine and L-Citrulline
2.1 Nitric Oxide Synthesis and Vascular Function
The Nitric Oxide Synthase Pathway Nitric oxide (NO) synthesis represents the most extensively studied function of L-arginine. Three distinct isoforms of nitric oxide synthase (NOS) catalyze the conversion of L-arginine to NO and L-citrulline: endothelial NOS (eNOS), neuronal NOS (nNOS), and inducible NOS (iNOS). Each isoform has unique regulatory mechanisms and tissue distributions, but all require L-arginine as substrate and several cofactors including NADPH, FAD, FMN, tetrahydrobiopterin (BH4), and calmodulin.
The reaction proceeds through a complex two-step oxidation process. First, L-arginine is hydroxylated to N-hydroxy-L-arginine, which is then oxidized to L-citrulline and NO. This process consumes 1.5 moles of NADPH and produces one mole of NO per mole of L-arginine. The reaction is highly dependent on the availability of BH4, which serves as a critical redox cofactor. When BH4 is deficient, NOS becomes “uncoupled,” producing superoxide instead of NO, which contributes to oxidative stress and endothelial dysfunction.
Vasodilation and Blood Pressure Regulation NO produced by endothelial cells diffuses into vascular smooth muscle cells, where it activates soluble guanylate cyclase (sGC). This enzyme converts GTP to cyclic GMP (cGMP), which activates protein kinase G (PKG). PKG then phosphorylates multiple targets that reduce intracellular calcium concentrations, leading to smooth muscle relaxation and vasodilation. This NO-sGC-cGMP pathway is the primary mechanism by which L-arginine and L-citrulline exert their blood pressure-lowering effects.
Clinical studies demonstrate that supplementation with either amino acid reduces systolic blood pressure by 5-8 mmHg and diastolic pressure by 2-5 mmHg in hypertensive patients. The effect is more pronounced in individuals with endothelial dysfunction, where baseline NO production is impaired. The vasodilatory effects extend beyond systemic blood pressure to include regional blood flow improvements in coronary, cerebral, renal, and peripheral vascular beds.
Endothelial Function and Anti-Atherogenic Effects Beyond vasodilation, NO exerts multiple anti-atherogenic effects. It inhibits platelet aggregation and adhesion to vascular walls, reducing thrombus formation. NO also suppresses the expression of adhesion molecules (VCAM-1, ICAM-1) and chemokines (MCP-1) that recruit inflammatory cells to the vascular endothelium. Additionally, it inhibits vascular smooth muscle cell proliferation and migration, key processes in atherosclerotic plaque development and restenosis after angioplasty.
L-Arginine and L-citrulline improve flow-mediated dilation (FMD), a gold-standard measure of endothelial function, by 30-50% in patients with cardiovascular risk factors. This improvement correlates with reduced levels of asymmetric dimethylarginine (ADMA), an endogenous NOS inhibitor. By restoring NO bioavailability, these amino acids reverse endothelial dysfunction—a critical early step in atherosclerosis and a predictor of cardiovascular events.
2.2 Metabolic Functions and Cellular Energetics
Creatine Synthesis and Energy Metabolism L-Arginine serves as a precursor for creatine synthesis, a compound essential for cellular energy storage and transfer. In the kidneys, L-arginine combines with glycine to form guanidinoacetate, catalyzed by L-arginine:glycine amidinotransferase (AGAT). Guanidinoacetate is then methylated by guanidinoacetate N-methyltransferase (GAMT) in the liver, using S-adenosylmethionine (SAM) as the methyl donor, to form creatine.
Creatine is phosphorylated to phosphocreatine in tissues with high energy demands, particularly skeletal muscle, cardiac muscle, and brain. Phosphocreatine serves as a rapid buffer for ATP regeneration during periods of high energy expenditure. The creatine kinase system catalyzes the reversible transfer of phosphate between phosphocreatine and ADP, maintaining cellular energy homeostasis. By supporting creatine synthesis, L-arginine indirectly influences cellular energetics, exercise performance, and neurological function.
Polyamine Production and Cell Proliferation L-Arginine is the primary precursor for polyamine synthesis—putrescine, spermidine, and spermine—via ornithine decarboxylase (ODC). Polyamines are organic cations essential for cell growth, proliferation, and differentiation. They stabilize DNA structure, modulate ion channels, and participate in protein synthesis. Polyamines are particularly important in rapidly dividing tissues, including intestinal mucosa, immune cells, and healing wounds.
The connection between L-arginine availability and polyamine synthesis has significant implications for tissue repair and regeneration. During wound healing, increased polyamine production supports fibroblast proliferation, collagen synthesis, and angiogenesis. Similarly, in the gastrointestinal tract, adequate L-arginine intake maintains mucosal integrity and supports the rapid turnover of intestinal epithelial cells.
Ammonia Detoxification and the Urea Cycle As integral components of the urea cycle, L-arginine and L-citrulline play critical roles in ammonia detoxification. Ammonia, primarily generated from amino acid catabolism and gut bacteria, is highly toxic to the central nervous system, causing cerebral edema, encephalopathy, and death if accumulated. The urea cycle converts ammonia to urea, which is then excreted by the kidneys.
L-Arginine serves as an allosteric activator of N-acetylglutamate synthase, which produces N-acetylglutamate, an essential activator of carbamoyl phosphate synthetase I—the rate-limiting enzyme of the urea cycle. Within the cycle, L-arginine is hydrolyzed by arginase to ornithine and urea, while L-citrulline is an intermediate metabolite. Supplementation with either amino acid enhances urea cycle flux, particularly in conditions of hyperammonemia such as liver cirrhosis, urea cycle disorders, and hepatic encephalopathy.
2.3 Immune Modulation and Anti-Inflammatory Effects
T-Cell Function and Immune Proliferation L-Arginine is essential for optimal T-cell function and immune response. It serves as a critical substrate for the expression of the CD3ζ chain of the T-cell receptor complex. When L-arginine availability is limited, such as in certain tumor microenvironments or chronic inflammatory conditions, CD3ζ expression is downregulated, impairing T-cell receptor signaling and reducing T-cell proliferation and cytokine production.
L-Arginine also influences T-cell differentiation. Adequate L-arginine levels promote the development of T-helper 1 (Th1) cells, which are crucial for cell-mediated immunity against intracellular pathogens and tumors. Conversely, L-arginine depletion favors the expansion of myeloid-derived suppressor cells (MDSCs) and regulatory T cells (Tregs), which have immunosuppressive effects. By maintaining L-arginine availability, supplementation can shift the balance toward effective anti-tumor and anti-pathogen immune responses.
Macrophage Function and Phagocytosis Macrophages utilize L-arginine through two competing pathways: inducible nitric oxide synthase (iNOS) and arginase. iNOS produces high levels of NO, which has antimicrobial and tumoricidal effects. Arginase converts L-arginine to ornithine and urea, supporting polyamine synthesis and cell proliferation. The balance between these pathways determines macrophage function—M1 macrophages express iNOS and produce NO for pathogen killing, while M2 macrophages express arginase and support tissue repair.
L-Citrulline supplementation can enhance macrophage function by replenishing L-arginine pools, particularly in conditions where arginase activity is elevated. This is particularly relevant in chronic infections and cancer, where MDSCs upregulate arginase, creating local L-arginine depletion that impairs macrophage function. By supporting the iNOS pathway, L-citrulline can enhance the antimicrobial and tumoricidal capacity of macrophages.
Cytokine Regulation and Inflammation Both amino acids modulate cytokine production and inflammatory responses. L-Arginine availability influences the balance between pro-inflammatory and anti-inflammatory cytokines. Adequate L-arginine levels reduce the production of pro-inflammatory cytokines such as TNF-α, IL-6, and IL-1β while increasing anti-inflammatory cytokines like IL-10. This effect is mediated through multiple mechanisms, including modulation of NF-κB signaling and mTOR pathway activity.
L-Citrulline exerts additional anti-inflammatory effects by reducing oxidative stress and enhancing NO bioavailability. NO suppresses leukocyte adhesion and migration into tissues, reducing inflammation at sites of injury or infection. Clinical studies have shown that L-citrulline supplementation reduces markers of systemic inflammation, including C-reactive protein (CRP) and IL-6, in patients with cardiovascular disease, obesity, and type 2 diabetes.
2.4 Hormonal Regulation and Endocrine Functions
Growth Hormone Secretion L-Arginine is a potent stimulator of growth hormone (GH) secretion from the anterior pituitary gland. This effect is mediated through multiple mechanisms, including suppression of hypothalamic somatostatin release and direct stimulation of pituitary somatotrophs. The GH response to L-arginine is dose-dependent, with intravenous infusion of 30 grams producing a 4-8 fold increase in plasma GH levels within 30-60 minutes.
Oral L-arginine supplementation also stimulates GH release, though to a lesser extent than intravenous administration. Doses of 5-9 grams taken before exercise or sleep can increase GH levels by 2-4 fold. The GH-releasing effect is enhanced when L-arginine is combined with exercise or other GH secretagogues like GABA. This property has led to the use of L-arginine in sports performance and anti-aging medicine, though the clinical significance of transient GH elevations remains debated.
Insulin Secretion and Glucose Metabolism L-Arginine influences pancreatic β-cell function and insulin secretion. It stimulates insulin release through multiple mechanisms, including membrane depolarization, closure of ATP-sensitive potassium channels, and increased intracellular calcium concentrations. Additionally, L-arginine-derived NO enhances glucose-stimulated insulin secretion by amplifying the insulin secretory response to glucose.
Beyond acute insulin secretion, L-Arginine improves insulin sensitivity in peripheral tissues. NO enhances glucose uptake in skeletal muscle and adipose tissue by promoting GLUT4 translocation to the cell membrane. Clinical studies have shown that L-arginine supplementation improves insulin sensitivity by 20-40% in patients with type 2 diabetes, obesity, and metabolic syndrome. This effect is associated with improved endothelial function and reduced inflammation.
Prolactin and Other Hormones L-Arginine also influences the secretion of other hormones, including prolactin, glucagon, and vasopressin. It stimulates prolactin release, particularly in women, though the physiological significance of this effect remains unclear. In the pancreas, L-arginine enhances glucagon secretion from α-cells, contributing to glucose homeostasis. In the hypothalamus, L-arginine-derived NO modulates the release of gonadotropin-releasing hormone (GnRH) and corticotropin-releasing hormone (CRH), influencing reproductive function and stress responses.
L-Citrulline, through its conversion to L-arginine, shares many of these hormonal effects. However, its more sustained pharmacokinetic profile may provide more stable modulation of hormone secretion compared to the transient effects of direct L-arginine supplementation.
3: Clinical Applications of L-Arginine and L-Citrulline
3.1 Cardiovascular Diseases
Hypertension Management Hypertension affects over one billion adults worldwide and is a leading risk factor for cardiovascular disease. Endothelial dysfunction and reduced NO bioavailability are central to its pathophysiology. L-Arginine and L-citrulline supplementation offer a promising therapeutic approach by restoring NO-mediated vasodilation and improving arterial compliance.
Meta-analyses of randomized controlled trials demonstrate that L-arginine supplementation (4-24 grams daily) reduces systolic blood pressure by 5.4 mmHg and diastolic pressure by 2.7 mmHg. The effect is more pronounced in patients with endothelial dysfunction, such as those with diabetes or established atherosclerosis. L-Citrulline shows comparable or superior efficacy, with studies showing 6 grams daily reducing systolic pressure by 7-8 mmHg and diastolic pressure by 3-4 mmHg. The combination of both amino acids (3 grams each daily) produces additive effects, reducing systolic pressure by 10-12 mmHg in hypertensive patients.
The mechanisms underlying these antihypertensive effects include improved endothelial function, reduced oxidative stress, decreased sympathetic nervous system activity, and modulation of renal sodium handling. Long-term studies (6-12 months) show sustained blood pressure reduction without evidence of tachyphylaxis, suggesting these amino acids may be suitable for chronic hypertension management.
Coronary Artery Disease and Angina In coronary artery disease (CAD), impaired NO production contributes to endothelial dysfunction, reduced coronary blood flow, and myocardial ischemia. L-Arginine supplementation improves endothelial function in patients with CAD, as measured by flow-mediated dilation (FMD) and coronary vasoreactivity.
Clinical trials in patients with stable angina show that L-arginine (6-9 grams daily) improves exercise tolerance, reduces the frequency of anginal attacks, and decreases ST-segment depression during exercise testing. These effects are attributed to enhanced coronary vasodilation, improved myocardial perfusion, and reduced platelet aggregation. Intravenous L-arginine has been used acutely to relieve refractory angina, producing rapid symptomatic improvement.
L-Citrulline shows similar benefits in CAD, with the advantage of more sustained plasma arginine elevation. Studies demonstrate that 6 grams of L-citrulline daily for 6 months improves myocardial perfusion defects on cardiac imaging and reduces angina symptoms in patients with microvascular angina. The combination of L-citrulline with other NO-boosting compounds like beetroot juice (rich in dietary nitrates) produces additive coronary vasodilatory effects.
Peripheral Artery Disease Peripheral artery disease (PAD) affects over 200 million people globally and is characterized by atherosclerotic occlusion of peripheral arteries, leading to reduced blood flow to the extremities. The most common symptom is intermittent claudication—leg pain during walking that is relieved by rest.
L-Arginine supplementation has been extensively studied in PAD. Multiple randomized trials demonstrate that 6-9 grams daily improves walking distance by 150-230% in patients with intermittent claudication. The mechanism involves enhanced NO production, leading to improved collateral circulation, reduced blood viscosity, and enhanced oxygen delivery to ischemic tissues. L-Arginine also improves endothelial function in the femoral artery and reduces markers of inflammation and oxidative stress in PAD patients.
L-Citrulline shows comparable efficacy in PAD, with the advantage of better tolerability and sustained effects. Studies using 3 grams of L-citrulline twice daily show significant improvements in walking distance, ankle-brachial index (ABI), and quality of life in PAD patients. The combination of L-citrulline with exercise training produces synergistic improvements in functional capacity, suggesting these interventions may be complementary in PAD management.
Heart Failure Heart failure (HF) affects over 60 million people worldwide and is associated with significant morbidity and mortality. Endothelial dysfunction, reduced NO bioavailability, and impaired skeletal muscle perfusion contribute to exercise intolerance and fatigue in HF patients.
L-Citrulline has emerged as a promising therapy for HF, particularly heart failure with preserved ejection fraction (HFpEF). Clinical trials show that 6 grams of L-citrulline daily for 8-12 weeks improves peak oxygen consumption (VO2 max) by 12-15%, enhances ventricular-arterial coupling, and reduces left ventricular filling pressures in HFpEF patients. These improvements are attributed to enhanced NO-mediated vasodilation, improved arterial compliance, and reduced systemic inflammation.
In heart failure with reduced ejection fraction (HFrEF), L-citrulline supplementation improves endothelial function, reduces pulmonary artery pressures, and enhances cardiac output during exercise. Studies also show reductions in N-terminal pro-B-type natriuretic peptide (NT-proBNP), a biomarker of cardiac wall stress. The combination of L-citrulline with standard HF therapy appears safe and may provide additional symptomatic and functional benefits.
3.2 Exercise Performance and Athletic Applications
Strength and Power Performance L-Citrulline has gained significant attention in sports nutrition for its ergogenic effects, particularly in strength and power activities. Multiple studies demonstrate that 8 grams of L-citrulline malate consumed 60 minutes before exercise increases repetitions to failure during resistance training by 20-25%. For example, trained athletes performing bench press showed an increase from 10.8 ± 2.1 to 13.2 ± 2.3 repetitions with L-citrulline supplementation compared to placebo.
The mechanisms underlying these performance benefits include enhanced ATP production via the malate-aspartate shuttle, improved phosphocreatine resynthesis, and increased blood flow to working muscles. L-Citrulline also reduces the sensation of fatigue during high-intensity exercise, allowing athletes to maintain higher training volumes and intensities.
L-Arginine supplementation shows more variable effects on strength performance, likely due to its poor bioavailability and rapid clearance. However, when combined with L-citrulline in a 1:2 ratio (arginine:citrulline), some studies report additive benefits for strength and power output, possibly due to more sustained NO production and creatine synthesis.
Endurance Performance For endurance athletes, L-citrulline supplementation enhances both aerobic capacity and performance. Studies in cyclists show that 6 grams consumed 2 hours before a time trial improves performance by 1.5-3%, equivalent to approximately 45-90 seconds over a 40-km time trial. Similarly, runners show improvements in 10-km race times by 2-3% with L-citrulline supplementation.
The endurance benefits are attributed to multiple mechanisms: enhanced oxygen delivery via vasodilation, improved mitochondrial efficiency, reduced oxygen cost of exercise, and enhanced ammonia clearance. L-Citrulline increases the rate of phosphocreatine resynthesis during recovery intervals, which is particularly beneficial for high-intensity intermittent sports like soccer, basketball, and tennis.
Long-term supplementation (6 grams daily for 4-8 weeks) produces additional adaptations, including increased mitochondrial density, enhanced oxidative enzyme activity, and improved lactate threshold. These chronic adaptations complement the acute ergogenic effects, making L-citrulline a valuable supplement for endurance athletes across training phases.
Recovery and Muscle Soreness One of the most consistent benefits of L-citrulline supplementation is its effect on recovery and delayed onset muscle soreness (DOMS). Studies show that 8 grams consumed daily for 7-10 days reduces muscle soreness by 30-40% at 24-72 hours post-exercise. This is accompanied by 25-35% reductions in creatine kinase (CK) and lactate dehydrogenase (LDH), biomarkers of muscle damage.
The mechanisms for improved recovery include enhanced blood flow and nutrient delivery to damaged muscles, reduced inflammation and oxidative stress, and accelerated clearance of metabolic byproducts like ammonia and lactate. L-Citrulline also stimulates muscle protein synthesis via mTOR pathway activation, potentially accelerating tissue repair and remodeling.
L-Arginine shows similar but less pronounced effects on recovery, likely due to its pharmacokinetic limitations. However, the combination of L-citrulline with other recovery-promoting nutrients like branched-chain amino acids (BCAAs) and tart cherry juice produces synergistic benefits for reducing muscle damage and soreness.
3.3 Metabolic Health and Diabetes
Type 2 Diabetes Management Type 2 diabetes affects over 400 million people worldwide and is characterized by insulin resistance, β-cell dysfunction, and endothelial impairment. L-Arginine and L-citrulline offer multiple benefits for diabetes management through their effects on insulin sensitivity, endothelial function, and glucose metabolism.
Clinical trials demonstrate that L-arginine supplementation (3 grams three times daily) improves insulin sensitivity by 30-40% in type 2 diabetic patients, as measured by hyperinsulinemic-euglycemic clamps. This is accompanied by reductions in fasting glucose (15-20 mg/dL) and HbA1c (0.5-1.2%). The mechanisms include enhanced NO-mediated GLUT4 translocation, improved pancreatic blood flow and β-cell function, and reduced inflammation.
L-Citrulline shows comparable or superior efficacy in diabetes, with studies showing 3 grams twice daily improving insulin sensitivity by 35-45% and reducing HbA1c by 0.6-1.3%. The advantage of L-citrulline lies in its more sustained effects and better tolerability. Long-term studies (6-12 months) show sustained improvements in glycemic control without evidence of diminishing effects.
Diabetic Complications Diabetic complications, including nephropathy, retinopathy, and neuropathy, are major causes of morbidity and mortality. Endothelial dysfunction and reduced NO bioavailability contribute significantly to the development and progression of these complications.
In diabetic nephropathy, L-arginine supplementation (6 grams daily) reduces albuminuria by 30-40% and slows the decline in glomerular filtration rate (GFR). These effects are attributed to improved renal hemodynamics, reduced oxidative stress, and decreased inflammation. Similarly, in diabetic retinopathy, L-arginine improves retinal blood flow and reduces markers of vascular inflammation, potentially slowing disease progression.
For diabetic neuropathy, both L-arginine and L-citrulline show benefits in improving nerve conduction velocity and reducing neuropathic pain. Studies using 3 grams of L-citrulline twice daily show significant improvements in neuropathy symptom scores and nerve function tests. The mechanisms include enhanced vasa nervorum blood flow, reduced oxidative stress, and improved neuronal NO signaling.
Obesity and Metabolic Syndrome Metabolic syndrome, characterized by abdominal obesity, insulin resistance, hypertension, and dyslipidemia, affects over 20% of adults globally. L-Arginine and L-citrulline target multiple components of this syndrome through their effects on endothelial function, insulin sensitivity, and lipid metabolism.
Clinical trials in obese individuals show that L-citrulline supplementation (6 grams daily for 8 weeks) reduces visceral adiposity by 8-12%, improves insulin sensitivity by 25-35%, and reduces systolic blood pressure by 5-7 mmHg. These changes are accompanied by favorable shifts in lipid profiles, including reductions in triglycerides (15-20%) and increases in HDL cholesterol (5-10%).
The mechanisms for these metabolic benefits include enhanced fat oxidation, reduced inflammation in adipose tissue, improved mitochondrial function, and modulation of appetite-regulating hormones. L-Citrulline also increases energy expenditure during physical activity, contributing to its weight management effects. When combined with lifestyle interventions (diet and exercise), L-citrulline produces additive benefits for weight loss and metabolic health improvement.
3.4 Reproductive Health and Sexual Function
Erectile Dysfunction Erectile dysfunction (ED) affects over 150 million men worldwide and is strongly associated with endothelial dysfunction and reduced NO bioavailability. The penile erection process requires NO-mediated relaxation of cavernosal smooth muscle, making L-arginine and L-citrulline logical therapeutic options.
Clinical studies show that L-arginine supplementation (1.5-5 grams daily) improves erectile function in men with mild-to-moderate ED. A meta-analysis of randomized trials found that L-arginine increases the International Index of Erectile Function (IIEF-5) score by 2-3 points compared to placebo, with response rates of 30-40%. The effects are more pronounced in men with endothelial dysfunction rather than neurogenic or psychogenic ED.
L-Citrulline shows superior efficacy for ED, with studies demonstrating that 1.5 grams daily increases erection hardness scores by 50% and improves IIEF-5 scores by 4-5 points. In a landmark study, 50% of men taking L-citrulline reported improvement in erection hardness compared to only 8.3% with placebo. The advantage of L-citrulline lies in its better pharmacokinetics and more sustained elevation of plasma arginine levels.
The combination of L-citrulline with other natural compounds like Pycnogenol (French maritime pine bark extract) produces synergistic effects for ED. Studies show that this combination improves erectile function in 80% of men with mild ED, with effects comparable to low-dose PDE5 inhibitors like sildenafil.
Female Sexual Dysfunction Female sexual dysfunction (FSD) is a complex disorder affecting desire, arousal, orgasm, and pain. While less studied than male ED, endothelial dysfunction and reduced blood flow to genital tissues contribute significantly to arousal disorders.
Preliminary studies suggest that L-citrulline may benefit women with FSD, particularly arousal disorders. A study in postmenopausal women showed that 3 grams of L-citrulline daily for 4 weeks improved desire, arousal, and satisfaction scores. The mechanisms include enhanced clitoral and vaginal blood flow, increased vaginal lubrication, and improved endothelial function.
L-Arginine has also been studied in combination with other nutrients for FSD. A formulation containing L-arginine, ginseng, and ginkgo biloba showed improvements in sexual desire and satisfaction in premenopausal women with FSD. However, more robust clinical trials are needed to establish the efficacy of these amino acids for female sexual health.
Fertility and Pregnancy Outcomes Both amino acids play important roles in reproductive physiology and pregnancy outcomes. L-Arginine is critical for placental development, uterine blood flow, and fetal growth. It promotes trophoblast invasion and spiral artery remodeling, processes essential for establishing adequate placental perfusion.
Clinical studies in pregnancy show that L-arginine supplementation (3 grams three times daily) reduces the risk of preeclampsia by 50-70% in high-risk women. It also improves fetal growth parameters and reduces the incidence of small-for-gestational-age births. The mechanisms include enhanced placental blood flow, reduced oxidative stress, and improved endothelial function in the uteroplacental circulation.
L-Citrulline shows similar benefits for pregnancy outcomes, with the advantage of better tolerability. Studies in animal models demonstrate that L-citrulline improves fetal growth and placental efficiency, though human clinical trials are limited. Both amino acids appear safe during pregnancy when used at recommended doses under medical supervision, though more long-term safety data are needed.
4: Synergistic Effects and Combined Supplementation