H. pylori Symptoms: From Chronic Gastritis to Increased Cancer Risk
Helicobacter pylori, often abbreviated as H. pylori, is a name that might sound unfamiliar to many, yet this spiral-shaped bacterium is one of the most common human pathogens in the world. It is a master of survival, uniquely adapted to thrive in one of the most hostile environments in the human body: the acidic lining of the stomach. For decades, the medical community believed that the stomach’s sterile, highly acidic environment was impervious to bacterial colonization. The discovery of H. pylori and its link to gastric illnesses not only overturned this long-held belief but also revolutionized our understanding and treatment of common digestive disorders. This comprehensive guide delves into the world of Helicobacter pylori, exploring its nature, how it infects and causes disease, the telltale signs and symptoms to watch for, the methods used to diagnose it, the arsenal of medical treatments available, the role of natural remedies and lifestyle changes in managing the infection, and answers to the most frequently asked questions about this pervasive bacterium.
The Unseen Invader: Understanding Helicobacter pylori
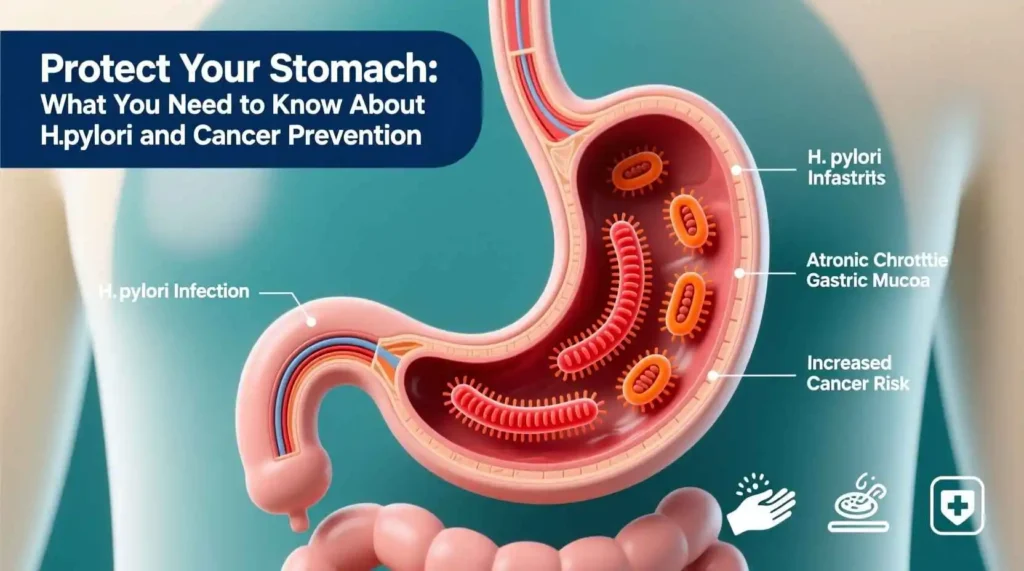
Helicobacter pylori is a Gram-negative, microaerophilic bacterium, meaning it requires oxygen to survive but at lower concentrations than found in the atmosphere. Its most striking feature is its shape: a delicate spiral or corkscrew form, which is key to its ability to colonize the stomach. This shape, combined with multiple powerful flagella (tail-like appendages), allows the bacterium to drill through the thick, protective mucus gel layer that coats the stomach lining, a barrier that was once thought to be impenetrable. Once it has burrowed through this mucus layer, H. pylori takes up residence on the surface of the epithelial cells that line the stomach, finding a niche where it is protected from the lumen’s harsh acidity.
The true genius of H. pylori’s survival lies in its production of an enzyme called urease. The stomach’s primary defense is its secretion of hydrochloric acid, creating a pH of around 1.5 to 3.5, a level so acidic it can dissolve metal. Urease is H. pylori’s secret weapon against this hostile environment. The enzyme acts on urea, a chemical naturally present in gastric juice, breaking it down into ammonia and carbon dioxide. The ammonia produced is alkaline and neutralizes the acid immediately surrounding the bacterium, effectively creating a small, protective cloud of neutral pH that allows it to survive. This neutralization not only protects the bacterium but also damages the protective mucus layer, making it more susceptible to acid and pepsin, a digestive enzyme.
The discovery of H. pylori is a story of scientific perseverance and a challenge to established dogma. In the early 1980s, two Australian scientists, Dr. Barry Marshall and Dr. Robin Warren, observed these curved bacteria in the stomach biopsies of patients with chronic gastritis and peptic ulcers. At the time, the prevailing medical wisdom was that ulcers were caused by stress, spicy food, and excess stomach acid. The idea that a bacterium could live in the stomach and cause these conditions was met with skepticism and ridicule from the medical establishment. In a now-famous act of self-experimentation to prove his theory, Dr. Marshall drank a broth containing cultured H. pylori. Within days, he developed severe gastritis, the inflammation of the stomach lining, which he then successfully treated with antibiotics. This dramatic demonstration, along with subsequent research, provided irrefutable evidence of the link between H. pylori and gastric disease. For their groundbreaking work, Marshall and Warren were awarded the Nobel Prize in Physiology or Medicine in 2005. Their discovery transformed the treatment of peptic ulcer disease from a chronic, recurring condition managed with acid-suppressing medication to a curable infection with a simple course of antibiotics.
H. pylori infection is incredibly common worldwide, making it one of the most widespread bacterial infections in humans. It is estimated that over half of the world’s population is infected with H. pylori. However, the prevalence varies dramatically depending on geographic location, socioeconomic status, and age. Infection rates are much higher in developing countries, where it can affect up to 80% of the population, often acquired during childhood. In contrast, the prevalence in developed countries is lower, typically between 20% and 50%, and is often associated with older age, poorer living conditions during childhood, or immigration from high-prevalence areas. The primary mode of transmission is believed to be from person to person, most likely through the oral-oral route (sharing saliva, via kissing, or sharing utensils) or the fecal-oral route (contaminated food or water). The fact that it is often acquired in childhood and can persist for life if untreated underscores the importance of understanding and managing this infection.
The Chain of Damage: How H. pylori Causes Disease
While the majority of individuals infected with H. pylori remain asymptomatic, meaning they experience no symptoms, the bacterium is a significant cause of various gastrointestinal diseases in a subset of those infected. The path from initial infection to clinical disease is a complex interplay between the virulence factors of the bacterium, the host’s immune response, and environmental factors.
The initial stage of H. pylori-related disease is chronic gastritis, which is an inflammation of the stomach lining. In virtually all infected individuals, H. pylori colonization leads to some degree of gastritis. The bacterium’s presence triggers a robust immune response. The body’s immune cells recognize H. pylori as a foreign invader and release a cascade of inflammatory chemicals, such as cytokines and chemokines, in an attempt to eliminate it. However, H. pylori has evolved mechanisms to evade and subvert this immune response, allowing it to persist. The result is a chronic, low-grade inflammation that can smolder for decades. This constant state of inflammation damages the epithelial cells of the stomach lining. While this initial gastritis may not cause any noticeable symptoms, it is the foundation upon which more serious diseases can develop.
For some individuals, this chronic gastritis can progress to a more severe condition called peptic ulcer disease. A peptic ulcer is an open sore that develops on the inside lining of the stomach (a gastric ulcer) or the upper portion of the small intestine (a duodenal ulcer). H. pylori is the cause of the vast majority of duodenal ulcers and a large percentage of gastric ulcers. The damage occurs through a multi-pronged attack. The ammonia produced by the bacterium’s urease enzyme directly damages the protective mucus layer, leaving the underlying stomach tissue vulnerable. The chronic inflammation further weakens this protective barrier. Additionally, H. pylori can also alter the regulation of stomach acid production. In some cases, particularly with duodenal ulcers, the infection can lead to an increase in acid production, which then bathes the now-unprotected duodenal lining in excess acid, creating an ulcer. The classic symptoms of a peptic ulcer include a burning or gnawing pain in the abdomen, bloating, heartburn, and nausea. The pain is often felt between the navel and the breastbone and may be temporarily relieved by eating or taking antacids, only to return when the stomach is empty.
In a smaller but significant number of cases, the long-term consequences of H. pylori infection can be much more severe. Chronic inflammation and cellular damage over many years can lead to changes in the cells of the stomach lining, a process known as metaplasia and dysplasia. These are precancerous changes that can, in a small fraction of infected individuals, eventually lead to the development of gastric cancer. H. pylori is now classified as a Group 1 carcinogen by the World Health Organization, meaning it is a definite cause of cancer in humans. It is the single strongest known risk factor for gastric adenocarcinoma, the most common type of stomach cancer. The journey from H. pylori infection to gastric cancer is a long and complex one, typically taking decades and involving a stepwise progression from chronic gastritis to atrophic gastritis (where the stomach glands are lost), intestinal metaplasia (where stomach cells are replaced by intestinal-type cells), dysplasia (abnormal, precancerous cells), and finally, invasive cancer. It is important to remember that while H. pylori is a major risk factor, the vast majority of infected people will never develop stomach cancer.
Another serious condition associated with H. pylori infection is MALT lymphoma, or mucosa-associated lymphoid tissue lymphoma. This is a rare type of non-Hodgkin’s lymphoma that arises in the stomach. The chronic immune stimulation caused by the persistent H. pylori infection can lead to the uncontrolled growth of lymphocytes, a type of white blood cell, in the stomach lining. Remarkably, in the early stages of this low-grade lymphoma, simply eradicating the H. pylori infection with antibiotics can cause the tumor to regress completely, demonstrating the direct causal link between the bacterium and this type of cancer.
The reason why some infected individuals remain asymptomatic while others develop ulcers or even cancer is not fully understood but is believed to be due to a combination of bacterial factors, host factors, and environmental influences. Certain strains of H. pylori are more virulent than others. The most well-studied virulence factors are the CagA (cytotoxin-associated gene A) and VacA (vacuolating cytotoxin A) proteins. Strains that possess the CagA gene, known as CagA-positive strains, are associated with a higher risk of developing peptic ulcers and gastric cancer compared to CagA-negative strains. The VacA protein can damage cells and suppress the immune response. Host genetic factors also play a role; variations in genes that control the immune response can make some individuals more susceptible to the damaging effects of the infection. Finally, environmental factors like smoking and a diet high in smoked, salted, or pickled foods can increase the risk of progression to more severe diseases.
Recognizing the Enemy: Symptoms of Helicobacter pylori Infection
One of the most challenging aspects of H. pylori is its ability to colonize the stomach for a lifetime without causing any noticeable symptoms in the majority of people. This silent nature means that many individuals are completely unaware they are infected. However, when symptoms do occur, they are often related to the conditions that H. pylori causes, primarily gastritis and peptic ulcer disease. The symptoms can be vague and easily mistaken for other common digestive issues, which can lead to delayed diagnosis and treatment.
The most common symptom associated with H. pylori infection is a persistent or recurrent pain or discomfort in the upper abdomen. This is often described as a burning, gnawing, or aching sensation. The location of the pain can sometimes offer a clue to the underlying problem. Pain from a gastric ulcer is often felt soon after eating, as food enters the stomach and stimulates acid production, which then irritates the ulcer. In contrast, pain from a duodenal ulcer is typically felt when the stomach is empty, such as between meals or during the night, and is often relieved by eating or taking antacids. However, these patterns are not always reliable, and the pain can sometimes be diffuse and difficult to pinpoint.
Bloating and a feeling of fullness after eating only a small amount of food are also frequent complaints. This is known as early satiety. The inflammation in the stomach lining can affect the stomach’s ability to expand and contract normally, leading to a sensation of bloating and discomfort. Nausea and sometimes vomiting can also occur, particularly if the gastritis is severe. In some cases, the vomit may contain blood, which can appear bright red or have the texture and color of coffee grounds. This is a serious sign indicating a bleeding ulcer and requires immediate medical attention.
Heartburn and acid reflux are also commonly reported by individuals with H. pylori. While H. pylori itself does not directly cause gastroesophageal reflux disease (GERD), the inflammation and changes in stomach acid regulation associated with the infection can exacerbate reflux symptoms in some people. It is a complex relationship, as some studies suggest that H. pylori infection, particularly certain strains, might actually have a protective effect against the development of severe GERD and its complications, like Barrett’s esophagus.
Unexplained weight loss can be another red flag, although it is less common. This can occur for several reasons. The discomfort associated with eating may lead to a reduced appetite and a conscious or unconscious avoidance of food. In more severe cases, the chronic inflammation can interfere with the body’s ability to absorb nutrients properly. Furthermore, unexplained weight loss is a symptom that must be taken seriously as it can also be a sign of more serious conditions, including stomach cancer.
In some cases, an H. pylori infection can manifest with less typical or “extra-digestive” symptoms. Some studies have suggested a possible link between H. pylori infection and iron-deficiency anemia or vitamin B12 deficiency. The chronic inflammation in the stomach can damage the cells that produce intrinsic factor, a protein necessary for the absorption of vitamin B12, leading to a condition called pernicious anemia. The inflammation can also cause minor, chronic bleeding in the stomach lining, which can lead to iron-deficiency anemia over time. There has also been research exploring potential links between H. pylori and certain skin conditions like chronic urticaria (hives) or rosacea, and even some neurological conditions, although these links are not yet firmly established and remain an area of active research.
It is crucial to emphasize that the presence of these symptoms does not automatically mean you have an H. pylori infection, as many other gastrointestinal conditions can cause similar complaints. However, if you experience persistent upper abdominal pain, unexplained nausea, bloating, or any of the more concerning symptoms like vomiting blood, black tarry stools (which can indicate bleeding in the digestive tract), or unexplained weight loss, it is essential to consult a healthcare professional for a proper evaluation and diagnosis.
Unmasking the Bacterium: Diagnosis of Helicobacter pylori
Diagnosing an H. pylori infection is a crucial step before initiating treatment. The decision to test for H. pylori is typically based on the patient’s symptoms, medical history, and risk factors. There are two main categories of diagnostic tests: invasive tests, which require an endoscopy to obtain a tissue sample from the stomach, and non-invasive tests, which can be performed without an endoscopic procedure. The choice of test depends on the individual patient’s situation.
Invasive tests are considered the gold standard for diagnosis and are typically performed during an upper gastrointestinal endoscopy, also known as a gastroscopy. During this procedure, a thin, flexible tube with a camera on the end is passed through the mouth, down the esophagus, and into the stomach and duodenum. This allows the doctor to directly visualize the lining of the stomach and duodenum to look for signs of inflammation, ulcers, or other abnormalities. If an ulcer or suspicious area is seen, the doctor can take small tissue samples, or biopsies, from the stomach lining. These biopsies can then be used in several ways to diagnose H. pylori.
The most common invasive test is the histological examination. The biopsy tissue is processed, stained, and examined under a microscope by a pathologist, who can identify the characteristic spiral-shaped H. pylori bacteria within the mucus layer and on the surface of the cells. This method is highly specific and also allows the pathologist to assess the degree of inflammation and any precancerous changes. Another test that can be performed on a biopsy sample is the rapid urease test (RUT), also known as the CLO test. This test exploits the bacterium’s urease enzyme. The biopsy sample is placed in a gel or solution containing urea and a pH indicator. If H. pylori is present, its urease will break down the urea into ammonia, which will change the pH of the solution, causing the indicator to change color, usually from yellow to pink or red. This provides a rapid result, often within hours. Finally, a biopsy sample can also be sent for a culture, where the bacteria are grown in a special laboratory medium. Culture is the most specific method and also allows for antibiotic susceptibility testing, which is crucial in cases of treatment failure, but it is more time-consuming, expensive, and not as widely available as the other methods.
Non-invasive tests are often the first-line choice for diagnosing H. pylori in patients without alarm symptoms (like unexplained weight loss or bleeding) who do not require an immediate endoscopy. These tests are simpler, less expensive, and avoid the discomfort and risks associated with an endoscopic procedure.
The most widely used non-invasive test is the urea breath test (UBT). This test is highly accurate and is based on the same principle as the rapid urease test. The patient drinks a solution containing urea that is labeled with a special, non-radioactive carbon isotope (either carbon-13 or carbon-14). If H. pylori is present in the stomach, its urease enzyme will break down the labeled urea, releasing the labeled carbon, which is then absorbed into the bloodstream and exhaled in the breath. The patient then breathes into a collection bag or device, and the amount of labeled carbon in the breath sample is measured. A high level indicates the presence of an active H. pylori infection. To ensure accuracy, patients must stop taking proton pump inhibitors (a type of acid-reducing medication) and antibiotics for a specified period (usually 1-2 weeks for PPIs and 4 weeks for antibiotics) before the test, as these medications can suppress the bacteria and lead to a false-negative result.
Another common non-invasive test is the stool antigen test (SAT). This test detects the presence of H. pylori antigens (proteins from the bacterium) in a stool sample. The patient provides a small stool sample, which is then analyzed in a laboratory using specific antibodies that bind to H. pylori antigens. Like the urea breath test, it is a reliable method for diagnosing an active infection and is often used for both initial diagnosis and for confirming eradication after treatment. It also requires the patient to be off PPIs and antibiotics beforehand.
Blood tests can also be used to detect H. pylori, but they are not recommended for routine diagnosis of an active infection. These tests detect antibodies (IgG and IgA) that the body has produced in response to the infection. The main limitation of blood tests is that antibodies can remain in the bloodstream for years or even for life after the infection has been successfully eradicated. Therefore, a positive blood test cannot distinguish between a current, active infection and a past, resolved infection. For this reason, blood tests are primarily useful for epidemiological studies or in specific situations where other tests are not available, but they are not the preferred method for diagnosing an active infection that requires treatment.
The choice of which test to use depends on the clinical context. For a young patient with typical ulcer-like symptoms and no alarm features, a non-invasive test like the urea breath test or stool antigen test is usually the first step. If the test is positive, treatment can be initiated. For an older patient, or one with alarm symptoms like unexplained weight loss, difficulty swallowing, or signs of bleeding, an upper endoscopy is usually the first-line investigation. This allows for direct visualization of the stomach lining, diagnosis of any ulcers or other pathologies, and collection of biopsies for H. pylori testing and histological examination to rule out cancer.
Once a diagnosis of H. pylori infection is made and treatment is indicated, the goal is clear: complete eradication of the bacterium. Successful eradication heals the associated gastritis, promotes the healing of peptic ulcers, and significantly reduces the risk of ulcer recurrence and the development of gastric cancer. However, H. pylori is a challenging bacterium to eradicate. It lives in a difficult-to-reach niche, and its ability to develop resistance to antibiotics has made treatment increasingly complex. Treatment is not a simple matter of taking one pill; it involves a combination of medications taken for a specific period, typically 10 to 14 days.
The cornerstone of H. pylori treatment is a combination of at least two antibiotics, along with a medication that reduces stomach acid production. The acid-reducing medication serves two important purposes. First, it helps to alleviate the symptoms of gastritis and ulcers. Second, and more importantly for eradication, it creates a less acidic environment in the stomach. Many antibiotics are less effective in highly acidic conditions, so by raising the stomach’s pH, the acid-reducing medication helps the antibiotics work more effectively against the bacteria. The two main classes of acid-reducing medications used are proton pump inhibitors (PPIs), such as omeprazole, lansoprazole, and esomeprazole, and histamine-2 (H2) receptor blockers, though PPIs are generally preferred and more effective for this purpose.
For many years, the standard first-line therapy was known as triple therapy. This regimen typically consisted of a PPI, combined with two antibiotics: amoxicillin and clarithromycin. This combination was highly effective for a long time. However, in recent years, the global rise in clarithromycin resistance has led to a significant decline in the success rate of this traditional triple therapy in many parts of the world. As a result, treatment guidelines have evolved.
Currently, one of the most recommended first-line therapies, especially in regions with high clarithromycin resistance, is bismuth quadruple therapy. As the name suggests, this regimen includes four components: a PPI, bismuth subsalicylate, tetracycline, and metronidazole. Bismuth is a compound with antimicrobial properties that can help protect the ulcer crater and has a direct toxic effect on H. pylori. This combination has been shown to be highly effective even in areas with high rates of antibiotic resistance. The main drawback is that it involves taking more pills per day, which can affect patient compliance.
Another effective first-line option is concomitant therapy, which involves taking a PPI and three different antibiotics (amoxicillin, clarithromycin, and metronidazole) simultaneously for 10 to 14 days. The idea is that by using three antibiotics, the therapy can overcome potential resistance to any single one of them. This regimen has shown good success rates but also increases the risk of side effects due to the higher antibiotic load.
For patients who are allergic to penicillin (and therefore cannot take amoxicillin), the treatment options are adjusted. A common regimen in this case is a PPI combined with clarithromycin and metronidazole. However, this dual-antibiotic therapy is less effective than triple therapy, and its success is highly dependent on local resistance rates to both clarithromycin and metronidazole.
The success of any H. pylori treatment regimen is highly dependent on patient adherence. It is absolutely crucial that patients take all the medications exactly as prescribed, for the full duration of the course. Stopping the medication early or skipping doses can lead to treatment failure and can also contribute to the development of antibiotic-resistant bacteria, making future eradication attempts much more difficult. Common side effects of these antibiotic regimens include diarrhea, nausea, vomiting, abdominal pain, a metallic taste in the mouth, and darkening of the stool (from the bismuth). While these side effects can be unpleasant, they are usually manageable. Patients should be encouraged to complete the course but should also contact their doctor if the side effects are severe or intolerable.
Unfortunately, first-line treatment fails in a percentage of patients, often due to antibiotic resistance or poor adherence. If the first attempt at eradication is unsuccessful, a second-line treatment, known as salvage therapy, is required. The choice of salvage therapy depends on which antibiotics were used in the first-line regimen and the local resistance patterns. A common approach is to use a different combination of antibiotics. For example, if a patient failed a clarithromycin-based triple therapy, a bismuth quadruple therapy that uses different antibiotics (tetracycline and metronidazole) would be a logical next step. Levofloxacin-based triple therapy (a PPI, amoxicillin, and levofloxacin) is another option for salvage therapy, though resistance to levofloxacin is also increasing. In complex cases of multiple treatment failures, it may be necessary to perform endoscopy again to obtain a culture and antibiotic susceptibility testing to tailor the treatment to the specific strain of H. pylori.
After completing a course of treatment, it is essential to confirm that the eradication was successful. This is known as “test-and-treat” followed by “test-of-cure.” The test-of-cure is typically performed at least four weeks after the completion of the antibiotic therapy and after the patient has been off PPIs for one to two weeks. The preferred tests for confirming eradication are the urea breath test or the stool antigen test, as they are non-invasive and highly accurate for detecting active infection. A blood test is not useful for this purpose because, as mentioned earlier, antibodies can persist long after the bacteria are gone. Confirming eradication is important to ensure the infection is gone, to allow for proper healing of ulcers, and to reduce the long-term risk of gastric cancer.
Harnessing Nature: Natural Remedies and Supportive Therapies